The purpose of this chapter is to provide background on basic scientific
aspects of human cloning for readers of this report. Background on stem
cell research is also included to enable readers to understand how cloned
embryos might be useful in stem cell and other biomedical research. This
limited treatment only summarizes and highlights basic aspects of these
topics, in part because two major detailed reports, Scientific and
Medical Aspects of Human Reproductive Cloning1
and Stem Cells and the Future of Regenerative Medicine,2
have been recently published.
This review is based largely on scientific research papers published through June 2002, supplemented by references to several articles in the popular press. However, the research areas of cloning and stem cell research are being very actively investigated, and significant new developments are published frequently. Publication of new results could change some of the interpretations and emphases in this review.
Use of unfamiliar technical terms has been avoided wherever possible. Scientific names and terms used are described and defined in the Glossary of Terms.
Some Basic Facts about Human Cell
Biology and Sexual Reproduction
We begin with some basic facts about human cells, germ cells (egg and sperm), and early embryonic development to provide the background for understanding the mechanism of cloning and the differences between sexual and asexual reproduction.
Normal human cells with nuclei contain forty-six chromosomes, twenty-two pairs plus two X chromosomes if the individual is female, or twenty-two pairs plus one X and one Y chromosome if the individual is male. These chromosomes contain nearly all of the cell's DNA and, therefore, the genes of the cell. During formation of sperm cells, a process of specialized cell division produces mature sperm cells containing twenty-three chromosomes (twenty-two unpaired chromosomes plus either X or Y). During the formation of eggs (oocytes), a process of specialized cell division produces a cell containing two pronuclei, each of which contains twenty-two unpaired chromosomes plus an X. During fertilization, a polar body containing one of these pronuclei is ejected from the egg.
Fusion of egg and sperm cells and the subsequent fusion of their nuclei (the defining acts of all sexual reproduction) produce a zygote that again contains a nucleus with the adult cell complement of forty-six chromosomes, half from each parent [See Figure 1]. The zygote then begins the gradual process of cell division, growth, and differentiation. After four to five days, the developing embryo attains the 100-200 cell (blastocyst) stage. In normal reproduction, the blastocyst implants into the wall of the uterus, where, suitably nourished, it continues the process of coordinated cell, tissue, and organ differentiation that eventually produces the organized, articulated, and integrated whole that is the newborn infant. According to some estimates, about half of all early human embryos fail to implant, and are expelled with the menses during the next menstrual cycle.
Not quite all the DNA of a human cell resides in its nucleus. All human cells, including eggs and sperm, contain small, energy-producing organelles called mitochondria. Mitochondria contain a small piece of DNA that specifies the genetic instructions for making several essential mitochondrial proteins. When additional mitochondria are produced in the cell, the mitochondrial DNA is replicated, and a copy of it is passed along to the new mitochondria that are formed. During fertilization, sperm mitochondria are selectively degraded inside the zygote. Thus, the developing embryo inherits solely or principally mitochondria (and mitochondrial DNA) from the egg.
Human reproduction has also been accomplished with the help of in vitro fertilization (IVF) of eggs by sperm, and the subsequent transfer of one or more early embryos to a woman for gestation and birth. Even though such union of egg and sperm requires laboratory assistance and takes place outside of the body, human reproduction using IVF is still sexual in the biological sense: the new human being arises from two biological parents through the union of egg and sperm.
Egg and sperm cells combined in vitro have also been used to start the process of animal development. Transfer of the resulting blastocysts into the uterus of a female of the appropriate animal species is widely used in animal husbandry with resulting successful live births.
Cloning (Asexual Reproduction) of Mammals
Cloning to produce live offspring carries with it several possibilities not
available through sexual reproduction. Because the number of presumably
identical donor cells is very large, this process could produce a very
large number of genetically virtually identical individuals, limited only
by the supply of eggs and female animals that could bear the young. In
principle, any animal, male or female, newborn or adult, could be cloned,
and in any quantity. Because mammalian cells can be frozen and stored
for prolonged periods at low temperature and grown again for use as donor
cells in cloning, one may even clone individuals who have died. In theory,
a clone could be cloned again, on and on, without limit. In mice, such
"cloning of clones" has extended out to six generations.4
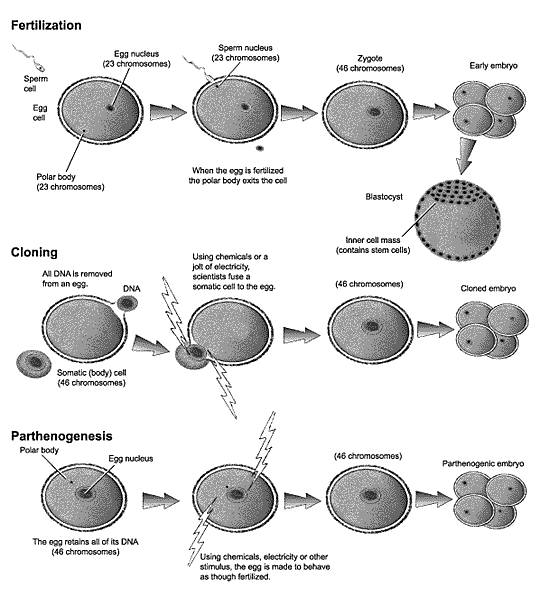
Figure 1: Diagram of early stages of human fertilization,
cloning, and parthenogenesis.
[Modified from Rick Weiss and Patterson
Clark, The Washington Post.]
Since the report of the birth of Dolly the cloned sheep, attempts have
been made to clone at least nine other mammalian species. As summarized
in Table 1, live offspring have been produced in a low percentage of cloned
embryo transfer experiments with sheep, cattle, goats, mice, pigs, cats5
and rabbits.6
According to a press report,7
attempts to clone rats, dogs, and primates using adult cell DNA
have not yet yielded live offspring. In experiments to clone different
mammalian species, many of the transferred cloned embryos fail to develop
normally and abort spontaneously in utero. In addition, a variety of health
problems have been reported in many of the cloned animals that survived
to live birth.8
However, some surviving cloned cattle appear physiologically similar to
their uncloned counterparts, and two cloned cows have given birth to their
own offspring.
9,10 