 |
|
|
|||||||||
|
|
||||||||||
Smallpox Vaccination and Adverse ReactionsGuidance for CliniciansPrepared by 1Bioterrorism Preparedness and Response
Program The material in this report originated in the National Center for Infectious Diseases, James M. Hughes, M.D., Director, and the Bioterrorism Preparedness and Response Program, Charles Schable, M.S., Acting Director; and the National Immunization Program, Walter A. Orenstein, M.D., Director, and the Epidemiology and Surveillance Division, Melinda Wharton, M.D., Director. Summary The guidance in this report is for evaluation and treatment of patients with complications from smallpox vaccination in the preoutbreak setting. Information is also included related to reporting adverse events and seeking specialized consultation and therapies for these events. The frequencies of smallpox vaccine-associated adverse events were identified in studies of the 1960s. Because of the unknown prevalence of risk factors among today's population, precise predictions of adverse reaction rates after smallpox vaccination are unavailable. The majority of adverse events are minor, but the less-frequent serious adverse reactions require immediate evaluation for diagnosis and treatment. Agents for treatment of certain vaccine-associated severe adverse reactions are vaccinia immune globulin (VIG), the first-line therapy, and cidofovir, the second-line therapy. These agents will be available under Investigational New Drug (IND) protocols from CDC and the U.S. Department of Defense (DoD). Smallpox vaccination in the preoutbreak setting is contraindicated for persons who have the following conditions or have a close contact with the following conditions: 1) a history of atopic dermatitis (commonly referred to as eczema), irrespective of disease severity or activity; 2) active acute, chronic, or exfoliative skin conditions that disrupt the epidermis; 3) pregnant women or women who desire to become pregnant in the 28 days after vaccination; and 4) persons who are immunocompromised as a result of human immunodeficiency virus or acquired immunodeficiency syndrome, autoimmune conditions, cancer, radiation treatment, immunosuppressive medications, or other immunodeficiencies. Additional contraindications that apply only to vaccination candidates but do not include their close contacts are persons with smallpox vaccine-component allergies, women who are breastfeeding, those taking topical ocular steroid medications, those with moderate-to-severe intercurrent illness, and persons aged <18 years. In addition, history of Darier disease is a contraindication in a potential vaccinee and a contraindication if a household contact has active disease. In the event of a smallpox outbreak, outbreak-specific guidance will be disseminated by CDC regarding populations to be vaccinated and specific contraindications to vaccination. Vaccinia can be transmitted from a vaccinee's unhealed vaccination site to other persons by close contact and can lead to the same adverse events as in the vaccinee. To avoid transmission of vaccinia virus (found in the smallpox vaccine) from vaccinees to their close contacts, vaccinees should wash their hands with warm soapy water or hand rubs containing >60% alcohol immediately after they touch their vaccination site or change their vaccination site bandages. Used bandages should be placed in sealed plastic bags and can be disposed of in household trash. Smallpox vaccine adverse reactions are diagnosed on the basis of clinical examination and history, and certain reactions can be managed by observation and supportive care. Adverse reactions that are usually self-limited include fever, headache, fatigue, myalgia, chills, local skin reactions, nonspecific rashes, erythema multiforme, lymphadenopathy, and pain at the vaccination site. Other reactions are most often diagnosed through a complete history and physical and might require additional therapies (e.g., VIG, a first-line therapy and cidofovir, a second-line therapy). Adverse reactions that might require further evaluation or therapy include inadvertent inoculation, generalized vaccinia (GV), eczema vaccinatum (EV), progressive vaccinia (PV), postvaccinial central nervous system disease, and fetal vaccinia. Inadvertent inoculation occurs when vaccinia virus is transferred from a vaccination site to a second location on the vaccinee or to a close contact. Usually, this condition is self-limited and no additional care is needed. Inoculations of the eye and eyelid require evaluation by an ophthalmologist and might require therapy with topical antiviral or antibacterial medications, VIG, or topical steroids. GV is characterized by a disseminated maculopapular or vesicular rash, frequently on an erythematous base, which usually occurs 6--9 days after first-time vaccination. This condition is usually self-limited and benign, although treatment with VIG might be required when the patient is systemically ill or found to have an underlying immunocompromising condition. Infection-control precautions should be used to prevent secondary transmission and nosocomial infection. EV occurs among persons with a history of atopic dermatitis (eczema), irrespective of disease severity or activity, and is a localized or generalized papular, vesicular, or pustular rash, which can occur anywhere on the body, with a predilection for areas of previous atopic dermatitis lesions. Patients with EV are often systemically ill and usually require VIG. Infection-control precautions should be used to prevent secondary transmission and nosocomial infection. PV is a rare, severe, and often fatal complication among persons with immunodeficiencies, characterized by painless progressive necrosis at the vaccination site with or without metastases to distant sites (e.g., skin, bones, and other viscera). This disease carries a high mortality rate, and management of PV should include aggressive therapy with VIG, intensive monitoring, and tertiary-level supportive care. Anecdotal experience suggests that, despite treatment with VIG, persons with cell-mediated immune deficits have a poorer prognosis than those with humoral deficits. Infection-control precautions should be used to prevent secondary transmission and nosocomial infection. Central nervous system disease, which includes postvaccinial encephalopathy (PVE) and postvaccinial encephalomyelitis (or encephalitis) (PVEM), occur after smallpox vaccination. PVE is most common among infants aged <12 months. Clinical symptoms of central nervous system disease indicate cerebral or cerebellar dysfunction with headache, fever, vomiting, altered mental status, lethargy, seizures, and coma. PVE and PVEM are not believed to be a result of replicating vaccinia virus and are diagnoses of exclusion. Although no specific therapy exists for PVE or PVEM, supportive care, anticonvulsants, and intensive care might be required. Fetal vaccinia, resulting from vaccinial transmission from mother to fetus, is a rare, but serious, complication of smallpox vaccination during pregnancy or shortly before conception. It is manifested by skin lesions and organ involvement, and often results in fetal or neonatal death. No known reliable intrauterine diagnostic test is available to confirm fetal infection. Given the rarity of congenital vaccinia among live-born infants, vaccination during pregnancy should not ordinarily be a reason to consider termination of pregnancy. No known indication exists for routine, prophylactic use of VIG in an unintentionally vaccinated pregnant woman; however, VIG should not be withheld if a pregnant woman develops a condition where VIG is needed. Other less-common adverse events after smallpox vaccination have been reported to occur in temporal association with smallpox vaccination, but causality has not been established. Prophylactic treatment with VIG is not recommended for persons or close contacts with contraindications to smallpox vaccination who are inadvertently inoculated or exposed. These persons should be followed closely for early recognition of adverse reactions that might develop, and clinicians are encouraged to enroll these persons in the CDC registry by calling the Clinician Information Line at 877-554-4625. To request clinical consultation and IND therapies for vaccinia-related adverse reactions for civilians, contact your state health department or CDC's Clinician Information Line (877-554-4625). Clinical evaluation tools are available at http://www.bt.cdc.gov/agent/smallpox/vaccination/clineval. Clinical specimen-collection guidance is available at http://www.bt.cdc.gov/agent/smallpox/vaccination/vaccinia-%20specimen-collection.asp. Physicians at military medical facilities can request VIG or cidofovir by calling the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) at 301-619-2257 or 888-USA-RIID. IntroductionSmallpox vaccine is made from live vaccinia virus and protects against the disease smallpox. It does not contain variola virus, the causative agent of smallpox (1). Because viral replication and shedding occurs at the vaccination site (beginning 2--5 days postvaccination), unintended transmission is possible from the time immediately after vaccination until the scab separates from the skin (approximately 2--3 weeks) (2). Although virus exists in the scab, it is bound in the fibrinous matrix, and the scab is not believed to be highly infectious. Viral shedding might be of shorter duration among revaccinees (2,3). During the smallpox eradication era, transmission usually required close interaction and occurred most often in the home (4) (see Transmission of Vaccinia Virus; see Preventing Contact Transmission). Worldwide, different vaccinia strains have been used for production of smallpox vaccine, but all U.S. vaccine formulations contain the New York City Board of Health (NYCBOH) vaccinia strain. This strain has been reported to be less reactogenic (i.e., it causes fewer adverse events) than other strains (1). U.S. National Pharmaceutical Stockpile (NPS) stores of smallpox vaccine include two previously manufactured calf-lymph--derived vaccines, Dryvax« (Wyeth Laboratories Inc., Marietta, Pennsylvania), and Aventis Pasteur vaccine (Swiftwater, Pennsylvania); and two newly developed vaccines from Acambis/Baxter Pharmaceuticals (Cambridge, Massachusetts), ACAM1000, which is grown in human embryonic lung cell culture (MRC-5), and ACAM2000, which is grown in African green monkey cells (VERO cells) (CDC Drug Services, unpublished data, 2002). Prospective studies are under way to determine the reactogenicity of the newer cell culture vaccines. Dryvax is the vaccine used in the current U.S. smallpox vaccination effort. CDC is holding other vaccines in reserve (5). Smallpox vaccination in the preoutbreak setting is contraindicated for persons who have the following conditions or have a close contact with the following conditions: 1) a history of atopic dermatitis (commonly referred to as eczema), irrespective of disease severity or activity; 2) active acute, chronic, or exfoliative skin conditions that disrupt the epidermis; 3) pregnant women or women who desire to become pregnant in the 28 days after vaccination; and 4) persons who are immunocompromised as a result of human immunodeficiency virus or acquired immunodeficiency syndrome, autoimmune conditions, cancer, radiation treatment, immunosuppressive medications, or other immunodeficiencies. Additional contraindications that apply only to vaccination candidates but do not include their close contacts are persons with smallpox vaccine-component allergies, women who are breastfeeding, those taking topical ocular steroid medications, those with moderate-to-severe intercurrent illness, and persons aged <18 years. In addition, history of Darier disease is a contraindication in a potential vaccinee and a contraindication if a household contact has active disease. In the event of a smallpox outbreak, outbreak-specific guidance will be disseminated by CDC regarding populations to be vaccinated and specific contraindications to vaccination. Normal Vaccination ProgressionSmallpox vaccine is administered by using the multiple-puncture technique with a bifurcated needle (6). The vaccinia virus replicates in the dermis of the skin; 3--5 days later, a papule forms at the vaccination site of immunocompetent vaccine-na´ve persons (also referred to as first-time or primary vaccinees) (1). The papule becomes vesicular (approximately day 5--8), then pustular, and usually enlarges to reach maximum size in 8--10 days. The pustule dries from the center outward and forms a scab that separates 14--21 days after vaccination, usually leaving a pitted scar (Figures 1--3). Formation by days 6--8 postvaccination of a papule, vesicle, ulcer, or crusted lesion, surrounded by an area of induration signifies a response to vaccination; this event is referred to as a major reaction or a take, and usually results in a scar. During the smallpox eradication era, persons with vaccination scars had much lower attack rates when exposed to smallpox cases than did nonvaccinated persons. Therefore, a take has been a surrogate correlate of immunity to smallpox. Although the level of antibody that protects against smallpox infection is unknown, >95% of first-time vaccinees (i.e., persons receiving their first dose of smallpox vaccine) have increased neutralizing or hemagglutination inhibition antibody titers (7). Interpreting Vaccination ResultsVaccination-site reactions are classified into two categories: major reactions and equivocal reactions (1). A major reaction indicates a successful vaccine take and is characterized by a pustular lesion or an area of definite induration or congestion surrounding a central lesion, which can be a scab or an ulcer. All other responses are equivocal reactions and are nontakes. Equivocal reactions can be caused by suboptimal vaccination technique, use of subpotent vaccine, or residual vaccinial immunity among previously vaccinated persons. Persons with equivocal reactions cannot be presumed to be immune to smallpox, and revaccination is recommended (Figures 4 and 5). The World Health Organization (WHO) has recommended that response to vaccination be evaluated on postvaccination day 6, 7, or 8 (1). These are the days of peak viral replication, and the period during which take should be assessed for both first-time vaccinees and revaccinees. If the response to vaccination is evaluated too early (e.g., <6 days postvaccination), certain equivocal responses will look reactive because of dermal hypersensitivity to vaccinial proteins. These reactions are sometimes referred to as immediate reactions but are not successful takes. If the response to vaccination is evaluated too late (e.g., >8 days postvaccination), the vaccination take might be missed among persons with prior immunity to vaccinia who might experience a more rapid progression of the vaccination site. Responses among revaccinees that resolve in <6 days are sometimes referred to as accelerated reactions and are not successful takes. Expected Range of Vaccine ReactionsA range of expected reactions occurs after vaccination. These normal reactions do not require specific treatment and can include fatigue, headache, myalgia, regional lymphadenopathy, lymphangitis, pruritis, and edema at the vaccination site, as well as satellite lesions, which are benign, secondary vaccinial lesions proximal to the central vaccination lesions (8) (Wyeth Laboratories. Dryvax [package insert]. Marietta, PA: Wyeth Laboratories, 1994). Historically, 21% of reactions associated with first-time vaccination caused the vaccinee to consult a physician (9). A recent vaccination trial was conducted among 680 adults, all of whom were first-time vaccinees (10). During the 14 days after vaccination, all reported having >1 of the following symptoms at some point: fatigue (50%), headache (40%), muscle aches and chills (20%), nausea (20%), and fever, defined as a temperature >37.7║C or 100║F (10%). Symptom duration was not reported. The majority of local symptoms were reported during the second week after vaccination and included pain at the vaccination site (86%), and regional lymphadenopathy (54%). Approximately one third of vaccinees were sufficiently ill to have trouble sleeping or to miss school, work, or recreational activities. Similar findings are reported by the CDC Smallpox Diary Card Database, a reporting system of postvaccination symptoms among 633 vaccinees who received smallpox vaccine during 2001--2002 (CDC, unpublished data, 2001--2002). In this series, postvaccination days 3--7 were the days when the majority of vaccinees (78%) reported their symptoms. In both series, symptoms were self-limited and required only symptomatic care. During the smallpox eradication era, fever after vaccination occurred frequently but was less common among adults than children (CDC, unpublished data). For adults, fever is more frequently noted among first-time vaccinees than revaccinees (NIH, unpublished data, 2003). In one vaccination series involving children, approximately 70% experienced >1 day of temperatures >100║F during the 4--14 days after primary vaccination (7), and 15%--20% of children experienced temperatures >102║F. After revaccination, 35% of children experienced temperatures >100║F, and 5% experienced temperatures of >102║F (11). Satellite lesions occasionally occur at the perimeter of the vaccination site and should not be confused with the early discrete vesicles that might coalesce into a central pox-like lesion. Satellite lesions are a benign finding, do not require treatment, and should be cared for as vaccination sites. (Figure 6). Large Vaccination Reactions and Robust TakesLarge vaccination reactions (i.e., >10 cm in diameter) at the site of inoculation occur in approximately 10% of first-time vaccinees and are expected variants of the typical evolution of the vaccination site (10). However, sometimes these large vaccination reactions have been reported as adverse events and misinterpreted as cellulitis, requiring antibiotic treatment. In the 1968 national surveillance of the United States for smallpox vaccine complications, 13 of 572 adverse event reports were for unusually large and painful robust takes (RTs) (9,12) (Figures 7 and 8). Bacterial infection of the vaccination site is uncommon but affects children more often than adults, because children are more likely to touch and contaminate their vaccination sites. In a 1963 U.S. national survey, 433 complications were reported among 14 million smallpox vaccinees; of these, two were secondary bacterial infections of the vaccination site (13). One case resolved without sequelae, whereas the other resulted in a nonfatal case of acute streptococcal glomerulonephritis. Other reports describe the occurrence of bacterial infection at the vaccination site, but do not provide details regarding the causative organisms (9,12). Specimens for bacterial cultures can be obtained by using swabs or aspiration. Gram stains can detect normal skin flora and are useful only when unusual pathogens are present. If empiric antibacterial therapy is administered, therapy should be adjusted after the bacterial pathogen and its sensitivities to various antibacterial medications are known. Identifying RTsDifferentiating an RT from bacterial cellulitis can be difficult. RTs occur 8--10 days postvaccination, improve within 72 hours of peak of symptoms, and do not progress clinically. Fluctuant enlarged lymph nodes are not expected and warrant further evaluation and treatment. In contrast, secondary bacterial infections typically occur within 5 days of vaccination or >30 days postvaccination, and unless treated, the infection will progress (14--16). The interval of onset to peak symptoms is the key factor in diagnosing RTs. Fever is not helpful in distinguishing RTs from bacterial cellulitis because it is an expected immunologic response to vaccinia vaccination. When an RT is suspected, management includes vigilant observation, patient education, and supportive care that includes rest of the affected limb, use of oral nonaspirin analgesic medications, as well as oral antipruritic agents. Salves, creams, or ointments, including topical steroids or antibacterial medications, should not be applied to the vaccination site. During 2001, CDC staff vaccinated 191 federal public health smallpox response team members; 9 vaccinees (5%) met the case definition for an RT, with an area of redness >7.5 cm with swelling, warmth, and pain at the vaccination site (CDC, unpublished data, 2002). Six vaccinees with RTs were treated for suspected bacterial cellulitis. Three affected vaccinees did not seek medical care and, therefore, did not receive antibiotic therapy. All affected vaccinees reported peak of symptoms 8--10 days after vaccination and improvement of symptoms within 24--72 hours whether they were treated with antibacterial medications. Cases did not cluster by age, sex, vaccination status, or vaccine lot number. To estimate an estimated rate of RTs, CDC staff conducted a limited survey and determined rates of 2% (2 of 99 persons) and 16% (13 of 80) (CDC, unpublished data, 2001). The different rates between clinics might be caused by different methods of case ascertainment. However, both clinics reported that irrespective of antibiotic therapy, symptoms peaked on postvaccination day 8--10, and improved within 24--72 hours. Antibacterial medications did not shorten the duration or lessen the severity of symptoms. Transmission of Vaccinia VirusVaccinia can be transmitted from a vaccinee's unhealed vaccination site to other persons by close contact and can lead to the same adverse events as in the vaccinee. Cases arising from contact transmission have resulted in either eczema vaccinatum (EV) or inadvertent inoculation, and these cases occurred approximately 5--19 days after suspected exposure to the index case (17). In addition, two cases have been reported of contact transmission, which resulted in fetal vaccinia (18,19) (see Fetal Vaccinia). No data exist to indicate that vaccinia transmission occurs by aerosolization (17). Although one study reported successful recovery of the vaccinia virus from the oropharynx of children receiving other vaccine strains (20), droplet infection has not been epidemiologically implicated in transmission of vaccinia. In one unpublished study in the 1960s (J. Michael Lane, M.D., formerly Director, Smallpox Eradication Program, Communicable Disease Center, personal communication, 2002), researchers were unable to recover the NYCBOH vaccinia strain from the nasal swabs of healthy vaccinees. The low rate of contact vaccinia and the link to direct physical contact indicate that aerosol transmission does not occur. The overall transmission of contact vaccinia in the 1960s occurred in the range of 2--6/100,000 first-time vaccinations (4); infection-control precautions should be taken to reduce this likelihood (21). Preventing Contact TransmissionCorrect hand hygiene prevents the majority of inadvertent inoculations and contact transmissions after changing bandages or other contact with the vaccination site (21). The vaccination site can be left uncovered or covered with a porous bandage (e.g., gauze) (6). Preventing Contact Transmission Among Health-Care Workers To prevent nosocomial transmission of vaccinia virus, health-care workers when involved in direct patient care should keep their vaccination sites covered with gauze or a similar material to absorb exudates that contain vaccinia. This dressing should be covered with a semipermeable dressing to provide a barrier to vaccinia virus. Using a semipermeable dressing alone is not recommended because it might cause maceration of the vaccination site and prolong irritation and itching, which subsequently leads to increased touching, scratching, and contamination of hands. If maceration of the vaccination site occurs, the lesion should be left open to air to allow the vaccination site to dry during a period that includes no direct contact with patients or other persons. The vaccination site should be covered during direct patient care until the scab separates (21). Administrative leave should be considered for health-care workers who are unable to adhere to the recommended infection-control measures, which require that vaccination sites be covered during patient care duties (21). Preventing Contact Transmission in Other Settings Transmission of vaccinia is also possible in other settings when close personal contact with children or other persons occurs. In these situations, the vaccination site should be covered with gauze or a similar absorbent material, and long-sleeved clothing should be worn. Careful attention should be paid to handwashing (21), which should be done with soapy warm water or hand-rub solutions that are >60% alcohol-based. Historically, the home was the setting where the majority of contact transmission occurred (4), presumably because of intimate contact and relaxed infection-control measures. Recognizing Vaccinia Virus TransmissionWhen evaluating a skin or other condition consistent with vaccinia, a history of smallpox vaccination and exposure to a household or close contact who has been vaccinated recently will often provide a source of the virus. A history of exposure to vaccinia might be difficult to obtain. A person might have had an inadvertent exposure and be unaware of being exposed to vaccinia virus, and rarely, persons have been deliberately inoculated by others as a way to vaccinate outside the approved vaccination programs (and possibly unwilling to acknowledge this exposure to vaccinia). In either case, clinicians should obtain a thorough medical history, including possible vaccinia exposure and risk factors for smallpox vaccine-related adverse reactions. Clinicians should counsel these patients regarding appropriate infection-control measures, care of their lesions, and when appropriate, the infectious risks incurred through deliberate inoculation of others. Follow-up of the patient and administration of appropriate treatment are critical if a vaccinia-related adverse reaction develops. In addition, these patients might be at increased risk for infection from bloodborne pathogens, and they should be counseled and treated appropriately. Adverse Reactions*Adverse reactions caused by smallpox vaccination range from mild and self-limited to severe and life-threatening (9,12,13,22,23). Certain smallpox vaccine reactions are similar to those caued by other vaccines (e.g., high fever, anaphylaxis, and erythema multiforme [EM]). Other adverse reactions specific to smallpox vaccination include inadvertent inoculation, ocular vaccinia, generalized vaccinia (GV), EV, progressive vaccinia (PV), postvaccinial encephalopathy (PVE) and encephalomyelitis (PVEM), and fetal vaccinia. Vaccinia-specific complications can occur among vaccinees or their contacts who have been inadvertently inoculated with vaccinia (3,7,24--26). The information regarding adverse events presented in this report is primarily based on reports from the 1960s. Although the vaccine remains unchanged, supportive care and therapeutic care options have improved. The U.S. population has also changed and now has a higher proportion of persons with contraindications to smallpox vaccination and who are at increased risk for adverse reactions. This group includes persons with atopic dermatitis (commonly referred to as eczema), or persons who are immunocompromised as a result of cancer, radiation, autoimmune conditions, immunosuppressive therapies, or immune deficiencies (e.g., human immunodeficiency virus [HIV] or acquired immunodeficiency syndrome [AIDS]). Updated reports regarding the frequency of adverse reactions will be disseminated by CDC as data become available. This guidance is for evaluation and treatment of patients with complications from smallpox vaccination administration during preoutbreak situations. In the event of a smallpox outbreak, considering smallpox disease will be necessary in the differential diagnosis of any recently vaccinated person who has an acute, generalized, vesicular, pustular rash illness. Until a determination is made regarding whether the rash is early smallpox disease or an adverse reaction to smallpox vaccine, these patients should be presumed to be highly infectious and placed in contact and respiratory isolation immediately. Appropriate local, state, and federal health and security officials should be contacted (5). Treatments available for specific complications of smallpox vaccination include vaccinia immune globulin (VIG), cidofovir, and ophthalmic antivirals (see Ocular Vaccinial Infections and Therapy). None of these therapies have been tested in controlled clinical trials for efficacy against vaccinial infection. However, because worldwide historical experience with using VIG to treat vaccinia-related adverse events exists, it is the first-line therapy. It is available in intravenous (IV) and intramuscular (IM) preparations under Investigational New Drug (IND) protocols through CDC and the U.S. Department of Defense (DoD). Cidofovir is an antiviral medication licensed for treatment of cytomegalovirus (CMV) retinitis among patients with AIDS. Cidofovir has been demonstrated to be nephrotoxic among humans and carcinogenic among animals. Cidofovir has never been used to treat vaccinia infections among humans. In animal models, cidofovir apparently protects against subsequent orthopoxvirus growth, if administered within 24 hours after experimental inoculation (27). However, no studies have demonstrated it to have an effect on orthopoxvirus infection after infection has been fully established. It will be available under IND protocols from CDC and DoD and should be considered second-line therapy for vaccinia complications (see Treatments). Frequencies of Adverse ReactionsTwo primary sources are available regarding the frequency of adverse reactions from NYCBOH smallpox vaccine: the 1968 U.S. national survey (12) and the 1968 10-state survey (9) (Table 1). These two studies used different methodologies, but are complementary. In the national survey, information was gathered from seven nationwide sources. The majority of the information concerning adverse reactions came from the American Red Cross VIG-distribution system. Reactions that did not require use of VIG and those for which VIG use was not warranted were less likely to be reported through this system. The national survey statistics should be considered minimal estimates of the risks from smallpox vaccination. In the 10-state survey, clinicians were actively contacted and urged to report all adverse reactions, including those considered less severe. For this reason, the 10-state survey data probably present a better estimate of the number of persons having adverse reactions. The range of frequencies for these two studies provides an estimate for the frequencies of adverse reactions that might be expected today (28) (Table 1). A review of vaccinia-related deaths (68) during a 9-year period (1959--1966 and 1968) revealed that deaths occurred among first-time vaccinees as a result of PVE (52%; 36 cases) and PV (28%; 19 cases) and among contacts as a result of EV (18%; 12 cases) (23). The strain of vaccinia virus might correlate with the type and frequency of adverse reactions (1,12). All U.S. preparations of smallpox vaccine contain the NYCBOH strain, one of the less reactogenic strains (1). Therefore, the U.S. experience might not represent international experience, which reflects use of other vaccinia strains. Virulence of vaccinia strain is associated with risk for PVE and PVEM, as well as the likelihood of contact transmission (1,4,17). Anticipated Adverse ReactionsAdverse reaction rates in the United States today might be higher than those previously reported because the proportion of persons at risk for adverse events is higher as a result of cancer, cancer therapy, radiation, immunomodulating medications, organ transplantation, and other illnesses (e.g., HIV/AIDS and eczema or atopic dermatitis). Adverse reactions might be better than previously expected because of advances in medical care. Rates for all adverse reactions are lower for persons previously vaccinated (4). During the smallpox eradication era, approximately two thirds of complications after smallpox vaccination might have been preventable and might have been avoided with better screening (13,29). However, screening will not eliminate risk, because the risk factors for certain adverse reactions have not been clearly defined and screening success is subject to recall bias and the participant's willingness to disclose personal information. Stringent medical screening of potential vaccinees for risk factors for adverse events, coupled with improved infection-control measures to prevent vaccinia transmission, will probably decrease preventable complications of vaccination. Common Adverse ReactionsLocal Skin Reactions Local skin reactions can occur after smallpox vaccination. These include allergic reactions to bandage and tape adhesives, RTs, and less commonly, bacterial infections of the vaccination site (4). Reactions to adhesives usually result in sharply demarcated lines of erythema that correspond to the placement of adhesive tape (Figures 9 and 10). Patients have local pruritis but no systemic symptoms and are otherwise well. Frequent bandage changes, periodically leaving the vaccination site open to air, or a change to paper tape might alleviate symptoms. Care should be used to vary the positioning of tape or bandages. This condition is self-limited and resolves when bandages are no longer needed. Topical and oral steroid treatment for this reaction should be avoided because the site contains live vaccinia virus. Salves, creams, or ointments, including topical antibacterial medications, should not be applied to the vaccination site. Nonspecific Rashes Common nonspecific rashes associated with smallpox vaccination include fine reticular maculopapular rashes, lymphangitic streaking, generalized urticaria, and broad, flat, roseola-like erythematous macules and patches (Figure11). These rashes are believed to be caused by immune response to vaccination and do not contain vaccinia. Erythematous or urticarial rashes can occur approximately 10 days (range: 4--17 days) after first-time vaccination. The vaccinee is usually afebrile, and the rash resolves spontaneously within 2--4 days (8). Nonspecific rashes are usually self-limited. These persons appear well and benefit from simple supportive care measures (e.g., oral anti-antihistamine agents). Dermatologic Manifestations of Hypersensitivity ReactionsEM, sometimes referred to as roseola vaccinia or toxic urticaria, might appear as different types of lesions, including macules, papules, urticaria, and typical bull's-eye (targetoid or iris) lesions (8,30). Because the number of clinical descriptions of vaccinia-associated EM rashes is limited, the following details are extrapolated from common descriptions of EM occurring after herpes simplex or mycoplasma infections. The hallmark target lesion of EM associated with other infections usually appears with a central, dark papule or vesicle, surrounded by a pale zone and a halo of erythema, usually within 10 days after viral infection (30). The limited clinical descriptions of EM after smallpox vaccination indicate that it follows a similar course (8). The rash of EM might be extremely pruritic, lasting <4 weeks, and patients benefit from administration of oral antipruritics (30) (Figure 12). Less commonly, hypersensitivity reactions can appear as a more serious condition, Stevens-Johnson syndrome (SJS). SJS can also arise from EM and typically includes systemic symptoms with involvement of >2 mucosal surfaces (31) or 10% of body surface area. This condition requires hospitalization and supportive care (30) (Figure 13). The role of systemic steroids for treatment of SJS is controversial; therefore, the decision to administer systemic steroids to patients with postvaccinial SJS should be made after consultation with specialists in this area (e.g., dermatologists, immunologists, or infectious disease specialists), according to the prevailing standard of care. VIG is not used to treat nonspecific rashes, EM, or SJS, because these lesions are probably a manifestation of a hypersensitivity reaction and are not believed to contain vaccinia virus. Vaccinia-Specific Adverse ReactionsThe following guidance related to recognizing, evaluating, and treating smallpox vaccine-related adverse reactions (Table 2). Inadvertent InoculationInadvertent inoculation is a common but avoidable complication of smallpox vaccination (9,22). Inadvertent inoculation occurs when vaccinia virus is transferred from a vaccination site to a second location on the vaccinee or to a close contact. The most common sites involved are the face, eyelid, nose, mouth, lips, genitalia, and anus (Figure 14). Among immunocompetent persons, lesions follow the same course as the vaccination site. Clinicians in the smallpox eradication era observed that when inadvertent inoculation of a vaccinee occurred close to the time of vaccination, the resulting secondary lesions matured at the same pace as the central lesion of the vaccination site. In contrast, lesions from inadvertent inoculation that occurred >5 days postvaccination appeared attenuated, which indicated that the developing immune response might limit the reaction (J. Michael Lane, M.D., formerly Director, Smallpox Eradication Program, Communicable Disease Center, personal communication, 2002) (22). A primary prevention strategy to avoid inadvertent inoculation is to instruct vaccinees and their close contacts to avoid touching or scratching the vaccination site from the time of vaccination until the scab separates. In addition, vigilant handwashing with soap and warm water or hand rubs containing >60% alcohol, after touching an unhealed vaccination site or changing a vaccination dressing is critical. Lesions from an inadvertent inoculation contain live vaccinia virus, and the same contact precautions necessary for a vaccination site are necessary for these secondary lesions. Persons at highest risk for inadvertent inoculation are younger persons (e.g., children aged 1--4 years) and those with disruption of the epidermis. Periocular and ocular implantation (hereafter referred to as ocular vaccinial disease) accounted for the majority of reported inadvertent inoculations and were often noted within 7--10 days of vaccination among first-time vaccinees (22,32). Ocular vaccinial disease can occur in different forms, including blepharitis (inflammation of the eyelid), conjunctivitis, keratitis (inflammation of the cornea, including epithelial and stromal forms), iritis, or combinations thereof (33) (Figures 15--19). When evaluating a patient with the new onset of a red eye or periocular vesicles, vaccinia infection should be considered and history of recent vaccinia exposure (e.g., smallpox vaccination or close contact with a vaccine recipient) should be sought. The goal of therapy of ocular disease is to prevent complications, including corneal scarring associated with keratitis (Figures 17 and 18), and the patient should be comanaged with an ophthalmologist. In a limited study of vaccinia keratitis among rabbits, 1 dose of VIG did not alter the clinical course, but rabbits treated with 5 daily doses (2.5--5 times that recommended for humans) developed larger and more persistent corneal scars, compared with control animals (34). The 2001 Advisory Committee on Immunization Practices (ACIP) recommendation states that VIG is contraindicated in a patient with vaccinial keratitis (6). However, in November 2002, this recommendation was reevaluated and modified by the Public Health Service (see Ocular Vaccinial Infections and Therapy). VIG should not be withheld if a comorbid condition exists that requires administration of VIG (e.g., EV or PV) and should be considered for severe ocular disease, except isolated keratitis. In these situations, VIG should be administered if the risk of the comorbid condition is greater than the potential risk of VIG-associated complications of keratitis (see Ocular Vaccinial Infections and Therapy). Uncomplicated inadvertent inoculation lesions are self-limited, resolving in approximately 3 weeks, and require no therapy. If extensive body surface area is involved, or severe ocular vaccinia infection (without keratitis) (Figure 19), or severe manifestation of inoculation has occurred, treatment with VIG can speed recovery and prevent spread of disease. Ocular Vaccinial Infections and Therapy Ocular vaccinial infections account for the majority of inadvertent inoculations. However, data upon which to base treatment recommendations are limited. Published reports of treatment of human infections are predominantly case series reports concerning clinical experience with older antiviral drugs (e.g., idoxuridine [IDU] or interferon) or VIG. These studies did not employ the prospective, randomized, double-blinded, controlled trials that are now standard; clinical details and follow-up information are often variable (35--38). None of the available topical ophthalmic antiviral agents have been studied among humans with ocular vaccinia disease, except in one case report, where vidarabine was apparently superior to IDU in treating blepharoconjunctivitis (38). Prophylaxis of the cornea with topical antiviral drugs is common ophthalmologic practice in treating ocular herpes simplex and varicella-zoster infections (33). Therapies that have been considered for treatment of ocular vaccinial infections include topical ophthalmic antiviral drugs (trifluridine [Viroptic,« King Pharmaceuticals, Inc., Bristol, Tennessee] and vidarabine [Vira-A,« King Pharmaceuticals, Inc., Bristol, Tennessee]) and parenteral VIG. Trifluridine and vidarabine are not approved by the Food and Drug Administration (FDA) for treatment of vaccinia disease, although the product labels for trifluridine and vidarabine state that the drugs have in vitro and in vivo activity against vaccinia virus. Vidarabine is no longer being manufactured, but supplies might be available in certain areas. Among humans with GV and EV, VIG treatment decreases size and limits extension of vaccinial lesions within 24--48 hours. Consequently, VIG has been considered a means to prevent spread of facial vaccinia to the eye and spread of ocular vaccinia without corneal involvement. No evidence exists that VIG is effective in treating vaccinial infection of the cornea (i.e., vaccinial keratitis). Case reports exist of human patients with vaccinial keratitis not treated with VIG who apparently experienced more severe sequelae (including corneal scarring and disciform edema) than described in case reports where VIG therapy was used (35,39--41), as well as a case report concerning use of VIGIM in treating vaccinial keratitis in which corneal scarring did not develop (41). Case reports indicated efficacy of VIGIM in treating vaccinial blepharoconjunctivitis and blepharitis (32,40,42). To discuss treatment options for ocular vaccinia, CDC convened a meeting of ophthalmology and infectious disease consultants in November 2002. On the basis of available data and input from these consultants, this report offers the following guidance for clinicians: Additional data from animal and human clinical studies are needed to improve the evidence base and to refine recommendations for ocular vaccinia disease. Physicians treating patients with ocular vaccinia infection are encouraged to enroll in studies designed to evaluate the safety and efficacy of VIG and available antiviral preparations for treatment of ocular complications. GVGV is characterized by a disseminated maculopapular or vesicular rash, frequently on an erythematous base, that usually occurs 6--9 days after first-time vaccination (1,8). The rash spans the spectrum of vaccinial lesions, from maculopapules to vesicles. Maculopapules can be mistaken for EM when they are accompanied by a substantial component of erythema (9) (J. Michael Lane, M.D., formerly Director, Smallpox Eradication Program, Communicable Disease Center, personal communication, 2002) (Figure 20). In other instances, the pearly vesicles of GV resemble the lesions of smallpox; however, GV does not follow the centrifugal distribution that is characteristic of smallpox (1) (Figure 21). GV rash might be preceded by fever, but usually, patients do not appear ill (Figure 22). Lesions follow the same course as the vaccination site. Lesions can be present anywhere on the body, including the palms and soles and can be numerous or limited. GV can appear as a regional form that is characterized by extensive satellite vesiculation around the vaccination site, or as an eruption localized to a body part (e.g., arm or leg), with no evidence of inadvertent inoculation (4) (Figure 23). A mild form of GV also exists, which appears with only a limited number of scattered lesions. The skin lesions of GV are believed to be spread by the hematogenous route (1) and might contain vaccinia virus. Therefore, contact precautions should be used when treating these patients. Patients should be instructed to keep lesions covered and avoid physical contact with others if their lesions are too numerous to cover with bandages or clothing. The differential diagnosis of GV includes EM, EV, inadvertent inoculation at multiple sites, and uncommonly, early stages of PV or other vesicular diseases (e.g., disseminated herpes or severe chickenpox). GV is self-limited among immunocompetent hosts. These patients appear well and do not require VIG, but might benefit from simple supportive care measures (e.g., nonsteroidal anti-inflammatory agents [NSAIDS] and oral antipruritics). VIG might be beneficial in the rare case where an immunocompetent person appears systemically ill. GV is often more severe among persons with an underlying immunodeficiency, and these patients might benefit from early intervention with VIG. EVEV is a localized or generalized papular, vesicular, or pustular rash, which can occur anywhere on the body, with a predilection for areas of previous atopic dermatitis lesions. Persons with a history of atopic dermatitis are at highest risk for EV. Onset of the characteristic lesions can be noted either concurrently with or shortly after the development of the local vaccinial lesions (1). EV cases resulting from secondary transmission usually appeared with skin eruptions approximately 5--19 days after the suspected exposure (1,17) (Figures 24 and 25). EV lesions follow the same dermatological course as the vaccination site in a vaccinee, and confluent lesions can occur (Figure 26). The rash is often accompanied by fever and lymphadenopathy, and affected persons are systemically ill (43). EV tends to be more severe among first-time vaccinees or unvaccinated contacts (12,44) (Figure 27). Atopic dermatitis, regardless of disease severity or activity, is a risk factor for experiencing EV among either vaccinees or their close contacts (21,22,44--46), but no data exist to predict the absolute risk for these persons. The majority of primary-care providers do not distinguish between eczema and atopic dermatitis when describing chronic exfoliative skin conditions, including among infants and young children (47,48). Animal studies demonstrate that an immunologic T-cell dysregulation predisposes persons affected with atopic dermatitis to disseminated progressive papular, vesicular, and pustular lesions, even in intact skin (47). EV can be associated with systemic illness that includes fever and malaise. Management includes hemodynamic support (e.g., as for sepsis) and meticulous skin care (e.g., as for burn victims). Patients might require volume repletion and vigilant monitoring of electrolytes as a result of disruption of the dermal barrier. Patients with EV are at risk for secondary bacterial and fungal infections of the lesions, and antibacterials and antifungals are indicated as necessary. One study determined that the mortality from EV was reduced from 30%--40% to 7% after the introduction of VIG (41). Therefore, establishing the diagnosis early not delaying treatment with VIG is imperative to reducing mortality. Patients are usually severely ill and can require multiple doses of VIG. Virus can be isolated from EV lesions, making these patients highly infectious. Infection-control precautions should be used to prevent secondary transmission and nosocomial infection (17). PVPV (also referred to as vaccinia necrosum, vaccinia gangrenosa, prolonged vaccinia, and disseminated vaccinia), is a rare, severe, and often lethal complication that occurs among persons with immunodeficiencies (43,49--51). This diagnosis should be suspected if the initial vaccination lesion continues to progress without apparent healing >15 days after smallpox vaccination (8). Anecdotal experience suggests that, despite treatment with VIG, persons with cell-mediated immune deficits have a poorer prognosis than those with humoral deficits (1). PV is characterized by painless progressive necrosis at the vaccination site with or without metastases to distant sites (e.g., skin, bones, and other viscera) (50) (Figure 28). The vaccination lesion does not heal, presumably secondary to an immune derangement, and progresses to an ulcerative lesion, often with central necrosis (9) (Figure 29). Initially, limited or no inflammation appears at the site, and histopathology can reveal absence of inflammatory cells in the dermis (52). During the weeks that follow, patients might experience bacterial infection and signs of inflammation (J. Michael Lane, M.D., formerly Director, Smallpox Eradication Program, Communicable Disease Center, personal communication, 2002). In a 1963 study, the majority of 66 cases initially reported to be PV were reclassified after follow-up as severe primary (i.e., major) reactions (22). Cases of severe major reactions cleared within 1--2 weeks without VIG treatment (Figures 30 and 31). With PV, vaccinia virus continues to spread locally and can metastasize to distant sites through viremia (Figure 32). Live vaccinia virus can be isolated from the skin lesions of these patients. Infection-control precautions, which include contact isolation, are required to avoid vaccinial infection of other persons and to limit risk for secondary infections. The differential diagnosis of PV includes severe bacterial infection, severe chickenpox, other necrotic conditions (e.g., gangrene), and disseminated herpes simplex infections. Persons at highest risk for PV include those with congenital or acquired immunodeficiencies, HIV/AIDS, cancer, and those on immunosuppressive therapies for organ transplantation or autoimmune disease. The degree and type of immunocompromise probably correlates with the risk for PV, although the protective level of cellular count or humoral immunity is unknown. Before the introduction of VIG and early antiviral medications, PV was universally fatal (23); but after VIG was used for PV treatment, the survival rate improved (9,13). Surgical debridement was used infrequently with variable success to treat the primary progressive necrotic lesions of PV (V. Fulginiti, M.D., Universities of Arizona and Colorado, personal communication, 2002). Management of PV should include aggressive therapy with VIG, intensive monitoring, and tertiary-level supportive care. Despite advances in medical care, PV probably will continue to be associated with a high mortality rate. Postvaccinial Central Nervous System DiseaseCentral nervous system (CNS) disease after smallpox vaccination is most common among infants aged <12 months and is a diagnosis of exclusion (12). Clinical symptoms reflect cerebral or cerebellar dysfunction with headache, fever, vomiting, altered mental status, lethargy, seizures, and coma (43). CNS lesions occur in the cerebrum, medulla, and spinal cord. Lumbar puncture can reveal an increased opening cerebral spinal fluid (CSF) pressure, and examination of CSF might indicate monocytosis, lymphocytosis, and elevated CSF protein (1,12,43). Both PVE and PVEM have been described (1). PVE typically affects infants aged <2 years and reflects cerebral damage as a result of vascular changes. Acute onset of symptoms occurs 6--10 days postvaccination and can include seizures, hemiplegia, aphasia, and transient amnesia. Associated histopathological changes include generalized cerebral edema, mild lymphocytic menigineal infiltration, widespread ganglion degenerative changes, and occasionally, perivascular hemorrhages. Patients can be left with cerebral impairment and hemiplegia (1). PVEM (or encephalitis) affects persons aged >2 years and includes abrupt onset of fever, vomiting, headache, malaise, and anorexia approximately 11--15 days after vaccination. Symptoms can progress to loss of consciousness, amnesia, confusion, disorientation, restlessness, delirium, drowsiness, seizures, and coma with incontinence or urinary retention, obstinate constipation, and sometimes menigismus. CSF, although under increased pressure, reveals normal chemistries and cell count. Histopathological features include perivenous demyelination and microglial proliferation in demyelinated areas with lymphocytic infiltration but limited cerebral edema. These pathological features are similar to what is observed in other postinfectious encephalitides (1,53). The strain of vaccinia virus used in smallpox vaccines might influence the frequency of PVE and PVEM (1). Reports based on European data indicate generally higher rates of PVE among persons vaccinated with non-NYCBOH strains (53). In the United States, where the principal strain used was the NYCBOH, the occurrence of PVE or PVEM was rare among first-time vaccinees (1,9,12). Unrelated diseases that cause encephalomyelitis or encephalopathy might be temporally related to smallpox vaccination (1). U.S. rates might include these unrelated events, artificially increasing the rates of PVE/PVEM (1,9). The pathophysiology of PVE/PVEM is not well understood, although an autoimmune process has been hypothesized (53,54). Vaccinia virus has been isolated from CSF and CNS tissue of affected persons (12,53,55). The significance of this finding is unknown in the absence of controlled trials that examine CSF of healthy vaccinees. No clinical criteria, radiographic findings, or laboratory tests are specific for the diagnosis of PVE. PVE/PVEM are diagnoses of exclusion, and other infectious or toxic etiologies should be considered before making these diagnoses. In the past, recipients of the NYCBOH strain who experienced PVE or PVEM had a 15%--25% mortality rate, and 25% of survivors were left with varying neurological deficits (12). No study has indicated that VIG can be an effective therapy for PVE or PVEM, and therefore, VIG is not recommended for treatment of PVE or PVEM. A prospective study of prophylactic use of VIG among Dutch army recruits demonstrated reduced incidence of PVE among persons vaccinated with a non-NYCBOH strain (56). This led to routine administration of VIG in first-time vaccinations of adults in the Netherlands (57). However, the incidence of PVE after smallpox vaccination with the NYCBOH strain is low (9); therefore, concomitant administration of VIG at time of vaccination has never been recommended with the NYCBOH strain. No specific therapy exists for PVE or PVEM; however, supportive care, anticonvulsants, and intensive care might be required. Because the clinical symptoms of PVE or PVEM are not believed to be a result of replicating vaccinia virus, the role of antivirals is unclear. Fetal VacciniaFetal vaccinia, resulting from vaccinial transmission from mother to fetus, is a rare, but serious, complication of smallpox vaccination during pregnancy or shortly before conception; <50 cases have been reported in the literature (58--60). Fetal vaccinia is manifested by skin lesions and organ involvement, and often results in fetal or neonatal death (61). The skin lesions in the newborn infant are similar to those of GV or PV and can be confluent and extensive (Figures 33 and 34). The number of affected pregnancies maintained until term is limited. Affected pregnancies have been reported among women vaccinated in all three trimesters, among first-time vaccinees as well as in those being revaccinated, and among nonvaccinated contacts of vaccinees (18,19). Because fetal vaccinia is so rare, the frequency of, and risks for, fetal vaccinia cannot be reliably determined. Whether virus infects the fetus through blood or by direct contact with infected amniotic fluid is unknown. No known reliable intrauterine diagnostic test is available to confirm fetal infection. Apart from the characteristic pattern of fetal vaccinia, smallpox vaccination of pregnant women has not been clearly associated with prematurity, low birth weight, and fetal loss. In addition, smallpox vaccine has not been demonstrated to cause congenital malformations (62--64). VIG might be considered for a viable infant born with lesions, although no data exist for determining the appropriate dosage or estimating efficacy. If a pregnant woman is inadvertently vaccinated or if she becomes pregnant within 4 weeks after vaccinia vaccination, she should be counseled regarding the basis of concern for the fetus. However, given the rarity of congenital vaccinia among live-born infants, vaccination during pregnancy should not ordinarily be a reason to consider termination of pregnancy. No indication exists for routine, prophylactic use of VIG for an unintentionally vaccinated pregnant woman; however, VIG should not be withheld if a pregnant woman experiences a condition where VIG is needed (e.g., EV). To expand understanding of the risk for fetal vaccinia and to document whether adverse pregnancy outcome might be associated with vaccination, CDC is establishing a prospective smallpox vaccination pregnancy registry (see Requests for Clinical Consultation and IND Therapies and for Registries Enrollment). Other Vaccine-Specific Adverse EventsLess frequently reported adverse events temporally associated with after smallpox vaccination include myocarditis, pericarditis (65--70), precipitation of erythema nodosum leprosum or neuritis among leprosy patients (1), and osteomyelitis (sometimes confirmed by recovery of vaccinia virus) (1,71). Reported skin changes at the vaccination scar have included malignant tumors (e.g., melanoma [8], discoid lupus [72], and localized myxedema as a symptom of Graves disease [73]). Reported neurologic complications after smallpox vaccination include transverse myelitis, seizures, paralysis, polyneuritis, and brachial neuritis (53,74). Whether these conditions are caused by smallpox vaccination or represent coincidental occurrences after vaccination is unclear. Temporal association alone does not prove causation (75). Other unknown adverse events after smallpox vaccination might yet be described. Determining causality of reported postvaccination events associated with a specific vaccine is challenging and requires careful weighing of all the scientific evidence, evaluation of the quality and consistency of the data, and consideration of biologic plausibility of the association between the vaccination and the event (Box 1) (76). Clinicians should report unexpected and clinically relevant adverse events after vaccination to the Vaccine Adverse Event Reporting System (VAERS) and follow local, state, and territorial reporting requirements (see Smallpox Adverse Event Reporting). Revaccination of Persons with History of Adverse EventsBefore the eradication of smallpox, clinicians were often faced with the decision of whether to revaccinate persons who had documented serious adverse reactions. One study recommended that persons with a history of postvaccinial CNS disease (e.g., PVE/PVEM) or PV should not be revaccinated. Revaccination of children who had EV was not contraindicated, although it was recommended that they receive VIG concomitantly. Revaccination of children with a history of inadvertent inoculation or erythematous or urticarial rashes presented no known or theoretical risk (8). Persons with a history of an adverse reaction to smallpox vaccination that leads to deferral should not knowingly be placed in a situation where they might be exposed to smallpox. No absolute contraindications exist regarding vaccination of persons with high-risk exposures to smallpox; persons at greatest risk for experiencing serious vaccination complications are also at greatest risk for death from smallpox. In this situation, the benefits of smallpox vaccination probably outweigh the risks for an adverse reaction from smallpox vaccine (6). Prophylaxis for Persons at High Risk Inadvertently Exposed to Vaccinia Virus Either Through Vaccination or Contact TransmissionHistorically, VIG was administered prophylactically to persons at increased risk for vaccine-related adverse events who required vaccination or who were inadvertently vaccinated (8). However, VIG administration is not without risk, and the efficacy of VIG as a prophylactic against vaccinial infection has not been studied in a controlled setting. Until VIG is evaluated for such use, VIG is not recommended for prophylaxis when persons with contraindications to smallpox vaccination are inadvertently exposed to vaccinia and are otherwise well. Such persons should have careful clinical follow-up to ensure prompt diagnosis and treatment of an adverse event, if one occurs. Furthermore, in the absence of circulating smallpox virus, VIG is not recommended for concomitant use with smallpox vaccination among persons with contraindications. As recommended by ACIP, careful screening criteria should be used to exclude persons with contraindications from preoutbreak smallpox vaccination programs (21). To better understand the risks for vaccinia exposure among persons with contraindications to smallpox vaccination, CDC plans to maintain a registry of inadvertent exposures among groups at high risk (e.g., vaccinee or contact with dermatologic or pregnancy contradications). Clinicians are encouraged to report these cases to CDC so that prompt treatment can be initiated when necessary, and patients can be followed by using a standardized protocol. These data will be used to assess risk for experiencing an adverse event and the potential role for prophylactic therapy among these patients (see Requests for Clinical Consultation and IND Therapies and for Registries Enrollment). Laboratory DiagnosticsClinical evaluation and a careful patient history of recent smallpox vaccination or contact with a recent vaccinee are the mainstays of diagnosis of smallpox vaccine-related adverse events. In situations where clinical diagnosis is not straightforward, laboratory diagnostics for vaccinia might be helpful and might prevent inappropriate use of potentially toxic therapies. However, diagnostics for conditions easily confused with vaccinia infection (i.e., varicella, herpes zoster, herpes simplex, and enteroviruses), should be considered first, in particular for a nonvaccinee or someone believed to be a noncontact of a vaccinee. Serologic testing for vaccinia is probably uninformative because it cannot be used to distinguish vaccinia immunity from vaccinia infection unless baseline antibody titers are available. Diagnostic tests for vaccinia include electron microscopy to identify presence of orthopoxvirus, and gene amplification (polymerase chain reaction [PCR]), and viral culture for vaccinia. Regarding vaccinia, these tests are available only for research purposes, but are undergoing multicenter validation studies that might enable FDA to approve the test reagents for diagnostic use. After that approval, testing will be made available through the Laboratory Response Network (LRN) (77), an extensive system of public health and private laboratories that can be accessed through consultation with state and local health departments. Consultation regarding appropriate use of specialized vaccinia laboratory testing will be available through CDC. Laboratory Specimen CollectionA suspected case of an adverse event after smallpox vaccination should be promptly reported to the appropriate local, state, or territorial health department. When appropriate, public health officials might recommend that clinical specimens be collected for further evaluation of a possible case. Specimen collection guidelines are available at http://www.bt.cdc.gov/agent/smallpox/vaccination/vaccinia-specimen-collection.asp. TreatmentsVIG, cidofovir, and topical ophthalmic antiviral drugs are among the therapies that can be used to treat adverse events after smallpox vaccination. Ophthalmic drugs are discussed elsewhere in this report (see Ocular Vaccinial Infections and Therapy). VIGVIG is a sterile solution of the immunoglobulin fraction of plasma, containing antibodies to vaccinia virus from persons who were vaccinated with smallpox vaccine. The available preparation of VIG is a previously licensed IM product (VIGIM) (produced by Baxter Healthcare Corporation in 1994) containing 0.01% thimerosal (a mercury derivative) as a preservative. Two new IV preparations (VIGIV) are in production and do not contain thimerosal. All preparations of VIG will be available as IND products through CDC and DoD. VIG has demonstrated efficacy in the treatment of smallpox vaccine adverse reactions that are secondary to continued vaccinia virus replication after vaccination (41,78). Such adverse reactions include EV, PV, or vaccinia necrosum, and severe cases of GV. VIG has no proven effectiveness for postvaccinia central nervous system disease. VIG is recommended for treating EV and PV. Because the majority of cases of GV are self-limited, VIG is recommended for treating GV only if the patient is seriously ill or has serious underlying disease that is a risk factor for a complication of vaccination (e.g., such immunocompromised conditions as HIV/AIDS). VIG can also be useful in treating ocular vaccinia that results from inadvertent implantation. When ocular vaccinia with keratitis is present, consideration of VIG should include the possible increased risk for corneal scarring (see Ocular Vaccinia Infections and Therapy) (Box 2). Side Effects VIG administration has been associated with mild, moderate, and severe adverse reactions. Mild adverse reactions include local pain and tenderness, swelling, and erythema at the injection site after IM administration of immunoglobulins and can persist from hours to 1--2 days after administration. Moderate adverse reactions include joint pain, diarrhea, dizziness, hyperkinesis, drowsiness, pruritis, rash, perspiration, and vasodilation. Back and abdominal pain, nausea, and vomiting can occur within the first 10 minutes of injection. Chills, fever, headache, myalgia, and fatigue can begin at the end of infusion and continue for hours. More severe reactions of this type might require pretreatment with corticosteroids or acetaminophen, if another dose of VIG is required. Serious adverse events associated with administration of VIGIV are expected to be similar to those observed with other intravenous immune globulin (IVIG) products, and can include hypotension, anaphylaxis and anaphylactoid systemic reactions, renal dysfunction, and aseptic meningitis syndrome (AMS). When AMS occurs, it usually begins from within hours to 2 days after treatment and can occur more frequently in association with high dosage (2 g/kg body weight) therapy. It is characterized by severe headache, nuchal rigidity, drowsiness, fever, photophobia, painful eye movements, nausea, and vomiting. Discontinuation of IVIG treatment has resulted in remission of AMS within days without sequelae. Anaphylaxis and anaphylactoid systemic reactions have been reported after IM or IV injection of human immunoglobulin preparations. The symptoms of classic anaphylactic reactions include flushing, facial swelling, dyspnea, cyanosis, anxiety, nausea, vomiting, malaise, hypotension, loss of consciousness, and in certain cases, death. Symptoms appear from within seconds to hours after infusion. The treatment of such reactions is immediate discontinuation of immune globulin and administration of epinephrine, oxygen, antihistamines, IV steroids, and cardiorespiratory support. When proteins prepared from human blood or plasma are administered, the potential for transmission of infectious agents cannot be totally excluded. This also applies to infectious agents that might not have been discovered or characterized when the current preparations of VIG were formulated. To reduce the risk of transmitting infectious agents, stringent controls are applied in the selection of blood and plasma donors, and prescribed standards are used at plasma-collection centers, testing laboratories, and fractionation facilities. VIG Risks and Contraindications Contraindications to VIG administration include an acute allergic reaction to thimerosal (for VIGIM) or a history of a severe reaction after administration of human immunoglobulin preparations. Persons with selective immunoglobulin A (IgA) deficiency might have antibodies to IgA and could have anaphylactic reactions to subsequent administration of blood products that contain IgA. In a rabbit model of vaccinia keratitis, substantial doses of VIG were associated with corneal scarring (34) (see Ocular Vaccinia Infections and Therapy). Whether VIG can cause fetal harm when administered to a pregnant woman or if it affects reproductive capacity is unknown. Although clinical experience with other preparations containing immunoglobulins indicates that no fetal adverse events result from immunoglobulins, no studies have evaluated the adverse effects of VIG on the fetus. VIG should be administered to a pregnant woman only if clearly needed. Similarly, whether VIG is excreted in breast milk is unknown; therefore, caution should be exercised when VIG is administered to a nursing woman. VIG is made from human plasma; therefore, a possible risk of transmission of viruses and a theoretical risk of transmission-adventitious agents that can cause Creutzfeldt-Jacob disease exist. The risk that these products contain infectious agents has been reduced by questioning plasma donors about risk factors for infection and by testing for the presence of certain viruses in the plasma. Furthermore, manufacturing processes have been validated for their ability to inactivate and remove viruses. Administration Detailed instructions regarding the administration of IM and IV VIG are included in the Investigator's Brochure portion of the IND materials that accompany the products. For treatment of vaccinial complications, the recommended dose of VIGIM (16.5% solution) is 0.6 mL/kg body weight (100 mg/kg body weight). VIGIM is to be administered intramuscularly, preferably in the buttock or the anterolateral aspect of the thigh. To reduce local pain and discomfort, dividing the dose into smaller volumes to be administered by multiple injections might be necessary (79). Because the concentration of the new VIGIV products differs from that of the IM preparation, clinicians should refer to the manufacturer's package insert, or IND protocol, for correct dosages. The dose for IV administration of VIG might range from 100 mg/kg body weight to 500 mg/kg body weight, depending on the VIGIV formulation. CidofovirCidofovir (Vistide,« Gilead Sciences, Foster City, California), a nucleotide analogue of cytosine, has demonstrated antiviral activity against certain orthopoxviruses in cell-based in vitro and animal model studies (80--82). Its effectiveness in the treatment of vaccinia-related complications among humans is unknown. Cidofovir has been demonstrated to be nephrotoxic among humans and carcinogenic among animals, even at low doses (Gilead Sciences. Cidofovir [Package insert]. Foster City, CA: Gilead Sciences, Inc; 2000). It is administered with probenecid and hydration. Cidofovir is approved by FDA for treating CMV retinitis among patients with AIDS. Its use for treating smallpox vaccination complications is recommended only under IND protocol sponsored by CDC. This IND is a research protocol to evaluate the clinical effect and outcomes of cidofovir as a secondary treatment of vaccinia-related complications that do not respond to VIG treatment. CDC will supply cidofovir at no cost for use under this IND protocol. Cidofovir will be released for civilian use by CDC and for military use by DoD, if 1) a patient fails to respond to VIG treatment; 2) a patient is near death; or 3) all inventories of VIG have been exhausted. This proposed use of cidofovir is investigational and has not been studied among humans; therefore, the benefit of cidofovir therapy for vaccinia-related complications is uncertain. Insufficient information exists to determine the appropriate dosing and accompanying hydration and dosing of probenecid if antiviral therapy is needed to treat smallpox vaccine-related adverse events among the pediatric age group. Dosages for these patients should be determined in consultation with specialists at CDC and DoD. Additional information regarding dosing and administration of cidofovir is included in the Investigator's Brochure that accompanies the release of this product to the clinician when cidofovir is used under the IND protocol. Side Effects The major complication of cidofovir therapy is renal toxicity, which is sometimes irreversible, results in renal failure, and requires dialysis to prevent death. To reduce the renal toxicity of cidofovir, it must be administered with careful IV hydration and with probenecid, a renal tubular blocking agent. Cidofovir has also been associated with neutropenia, proteinuria, decreased intraocular pressure/ocular hypotony, anterior uveitis/iritis, and metabolic acidosis. Cidofovir-related carcinogenicity, teratogenicity, and hypospermia have been reported in animal studies. Mammary adenocarcinomas developed in rats exposed to 0.04 times the human exposure at the dose used in clinical practice on the basis of area-under-the-curve comparisons (Gilead Sciences, Inc. Cidofovir [Package insert]. Foster City, CA: Gilead Sciences, Inc; 2000). Probenecid has been associated with headache, anorexia, nausea, vomiting, urinary frequency, hypersensitivity reactions, anemia, hemolytic anemia, nephritic syndrome, hepatic necrosis, gout, uric acid stones, and renal colic. Probenecid should be used with caution among children, pregnant women and persons with sulfa drug allergy (see manufacturer's package insert). Administration Details for administration of cidofovir are included with the medication and IND materials that are shipped by CDC. The proposed dose of cidofovir for treatment of vaccinia complications is 5 mg/kg body weight administered intravenously, one time, during a 60-minute period. A second dose 1 week later should be considered if no response occurs to the first dose. Dose adjustment might be needed to compensate for decreased excretion caused by renal dysfunction if a second dose is needed. Administration procedures include assessment of renal function and use of saline hydration, and probenecid, before and after cidofovir, according to the regimen specified in the IND protocol (and in the package insert for treatment of CMV retinitis). Patients who receive cidofovir should be followed closely, both for drug toxicities and for the outcome of their serious adverse reaction. IND protocols require viral cultures to monitor for emerging viral resistance to cidofovir. The protocol materials will be supplied to facilitate monitoring and information collection. Long-term follow-up is required under the IND protocol to monitor for carcinogenicity, renal insufficiency, and teratogenicity. Requests for Clinical Consultation and IND Therapies and for Registries EnrollmentIn October 2002, ACIP recommended that enhanced terrorism preparedness should include vaccination of smallpox public health response and health-care teams (21). Implementation of this vaccination program was determined to be the responsibility of the states and territories in conjunction with local predesignated hospitals. Before participation in the vaccination program, states and territories should establish a comprehensive program to manage vaccinees and their contacts who experience an adverse event after smallpox vaccination. Hospitals that participate should assign physicians with expertise in infectious diseases, neurology, dermatology, allergy/immunology, and ophthalmology to assess and manage adverse events among vaccinees and their contacts. Vaccinees and their affected contacts should have access to evaluation and medical care for a suspected adverse event 24 hours/day and 7 days/week. CDC will provide consultation to state and territorial public health officials, their surrogate providers, and other requesting physicians regarding recognition, evaluation, diagnosis, and treatment of adverse events after smallpox vaccination through an information line for clinicians that will be staffed 24 hours/day, 7 days/week. In addition, CDC will provide consultation for evaluation and care of persons with contraindications to smallpox vaccination that have an inadvertent exposure to vaccinia virus (e.g., vaccination of a pregnant woman or a person with atopic dermatitis). These persons also will be enrolled in a vaccination registry for prospective follow-up. Referring providers should complete a thorough vaccination history and physical examination on all patients with a suspected adverse event before accessing CDC's Clinician Information Line. In addition, high-resolution digital photographs of dermatological manifestations of adverse events can aid in the recognition of specific dermatological manifestations of adverse events and should be obtained with the patient's permission and forwarded whenever possible. Providers seeking assistance should first contact their state health department before accessing the CDC consultation service or requesting VIG or cidofovir (Box 3). To aid providers in discerning the presence or severity of vaccine-related complications, CDC has developed draft clinical evaluation tools to assist with expected adverse events. These clinical evaluation tools are available at http://www.bt.cdc.gov/agent/smallpox/vaccination/clineval; this website will be updated as additional information becomes available. Feedback regarding the utility of these clinical evaluation tools is requested and can be submitted by e-mail to spoxtool@cdc.gov. In addition, CDC and other U.S. Department of Health and Human Services agencies will collect data related to the frequency of smallpox vaccine adverse events and the clinical outcome of affected persons. These data will provide an update concerning the medical risks associated with smallpox vaccination and the efficacy and safety of INDs used in the treatment of adverse events. Smallpox Vaccine Adverse Event ReportingProviders are strongly encouraged to report serious adverse events to VAERS after the administration of the smallpox vaccine (Box 4). VAERS is a passive reporting system for safety monitoring of all vaccines licensed in the United States, and is jointly managed by CDC and FDA. CDC and FDA will monitor smallpox vaccine-related adverse event reports daily, and will provide enhanced surveillance of adverse events after administration of the smallpox vaccine. However, adverse events that are judged to be serious or unexpected and which require CDC consultation or IND therapies (VIG or cidofovir) should not be solely reported to VAERS. These cases should instead be immediately reported by phone to the appropriate state health department officials and CDC, who will assist the reporting provider with completion of a VAERS form. All other smallpox vaccine adverse events that are serious, but do not require CDC consultation or administration of IND therapies, should be reported directly to VAERS within 48 hours of recognition. All other adverse events should be directly reported to VAERS within 1 week (Box 4). Additional InformationCDC, in collaboration with the U.S. Department of Health and Human Services, has developed a website, which is available at http://www.bt.cdc.gov/training/smallpoxvaccine/reactions. Information and photographs related to smallpox vaccination, normal vaccination reactions, adverse events after vaccination, and treatments for adverse reactions can be located at this website. Acknowledgments The preparers are grateful for the review of staff from FDA and J. Michael Lane, M.D., formerly Director, Smallpox Eradication Program, Communicable Disease Center. In addition, the preparers acknowledge R. Dana Bradshaw, M.D., Uniformed Services University of the Health Sciences; Walla Dempsey, Ph.D., National Institutes of Health; Vincent Fulginiti, M.D., University of Arizona Health Sciences Center; Kathleen Fullerton, M.P.H., CDC; John D. Grabenstein, Ph.D., U.S. Army Medical Command; D.A. Henderson, M.D., U.S. Department of Health and Human Services; Stephen Heyse, M.D., National Institutes of Health; Niranjan Kanesa-Thasan, M.D., USARMRIID; Peter Laibson, M.D., Wills Eye Hospital; Philip LaRussa, M.D., Columbia University College of Physicians and Surgeons; Myron Levin, M.D., University of Colorado Health Sciences Center; Todd Margolis, M.D., Ph.D., University of California at San Francisco; Leroy Marklund, USAMRIID; Deborah Pavan-Langston, M.D., Harvard Medical School; Jay Pepose, M.D., Ph.D., Washington University School of Medicine; Janine Smith, M.D., National Institutes of Health; James Sprague, M.D., Georgetown University School of Medicine; Carol Tacket, M.D., University of Maryland School of Medicine; Melissa Taylor, CDC; Bruce C. Tierney, M.D., CDC; and Michael Zegans, M.D., Dartmouth Medical School, for their assistance. The preparers are also grateful for the assistance of the Clinical Immunization Safety Assessment (CISA) Network, which was launched in October 2001 as a National Immunization Program (NIP) initiative designed to investigate adverse events after vaccination through intensive prospective clinical evaluations. The CISA network includes clinical research-scientists at Boston Medical Center, Boston, Massachusetts; New York Presbyterian-Columbia Hospital, New York, New York; Johns Hopkins University, Baltimore, Maryland; the University of Maryland, Baltimore, Maryland; Vanderbilt University, Nashville, Tennessee; Stanford University, Stanford, California; and Kaiser Permanente Vaccine Study Center, Oakland, California. References
* An adverse reaction is an untoward effect that occurs after a vaccination and is extraneous to the vaccine's primary purpose of producing immunity. Adverse reactions have been demonstrated to be caused by the vaccination. Adverse reactions also are referred to as vaccine side effects or complications. In contrast, adverse events are untoward effects observed or reported after vaccinations, but a causal relation between the two have yet to be established. Therefore, adverse events include both 1) adverse reactions and 2) other events associated with vaccinations only by coincidence (i.e., they would have occurred also in the absence of vaccination). This report focuses on adverse reactions known to be caused by smallpox vaccine on the basis of extensive prior experience. Additional previously unknown adverse events might be reported with reintroduction of smallpox vaccinations; however, whether they are causally related will require additional evaluation. List of Abbreviations Used in This Report
Table 1 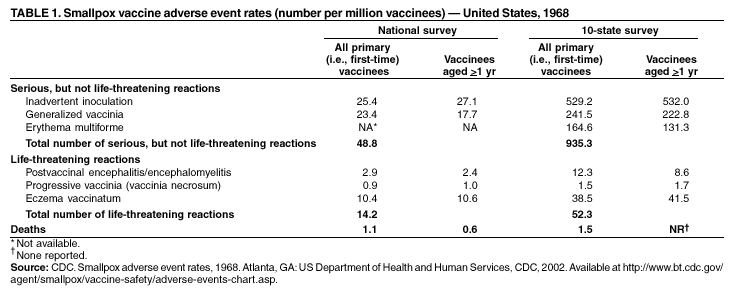 Return to top. Box 1 Box 3 Figure 5
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. Page converted: 1/23/2003 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|