
|
| Blood | Therapeutics | Vaccines | Cellular & Gene Therapy | Allergenics | Tissue | Devices |
Package Insert
Dryvax® (Smallpox Vaccine, Dried, Calf
Lymph Type)
Wyeth Laboratories, Inc.
Table of Contents
Description
Clinical Pharmacology
Indications and Usage
Contraindications
Precautions
Adverse Reactions
Dosage and Administration
How Supplied
Storage
References
Smallpox Vaccine
Dried, Calf Lymph Type
Dryvax®
Dried Smallpox Vaccine
Rx only
DO NOT INJECT INTRAMUSCULARLY (IM), INTRAVENOUSLY (IV), or SUBCUTANEOUSLY (SC). FOR CONVENTIONAL SMALLPOX VACCINATION (SCARIFICATION) ONLY.
Smallpox Vaccine, Dried, Calf Lymph Type, Dryvax® , is a live-virus preparation of vaccinia virus prepared from calf lymph. The calf lymph is purified, concentrated, and dried by lyophilization. During processing, polymyxin B sulfate, dihydrostreptomycin sulfate, chlortetracycline hydrochloride, and neomycin sulfate are added, and trace amounts of these antibiotics may be present in the final product. The reconstituted vaccine has been shown by appropriate test methods to contain not more than 200 viable bacterial organisms per mL.
The diluent for Dryvax® contains 50% glycerin, and 0.25% phenol in Sterile Water for Injection, USP.
The reconstituted vaccine, which contains approximately 100 million infectious vaccinia viruses per mL, is intended only for multiple-puncture use, ie, administration of the vaccine into the superficial layers of the skin using a bifurcated needle.
CLINICAL PHARMACOLOGY
Introduction of potent smallpox vaccine containing infectious vaccinia viruses into the superficial layers of the skin results in viral multiplication, immunity, and cellular hypersensitivity. With the primary vaccination, a papule appears at the site of vaccination on about the 2nd to 5th day. This becomes a vesicle on the 5th or 6th day, which becomes pustular, umbilicated, and surrounded by erythema and induration. The maximal area of erythema is attained between the 8th and 12th day following vaccination (usually the 10th). The erythema and swelling then subside, and a crust forms which comes off about the 14th to 21st day. At the height of the primary reaction known as the Jennerian response, there is usually regional lymphadenopathy and there may be systemic manifestations of fever and malaise.
Primary vaccination with product at a potency of 100 million pock-forming units (pfu)/mL elicits a 97% response rate by both major reaction (see DOSAGE AND ADMINISTRATION, Interpretation of Responses: Major Reaction) and neutralizing antibody response in children.1,2 Immunity wanes after several years, and an allergic sensitization to viral proteins can persist. This allergy is manifested by the appearance of a papule and a small area of redness appearing within the first 24 hours after revaccination; this may be the maximum reaction but not infrequently vesicles appear in 24 to 48 hours with ultimate scabbing. The peak of this type of reaction is passed within three days following the application of fully potent vaccine with an antibody rise occurring in roughly half of those who exhibit such a reaction. As immunity wanes, revaccination with potent vaccine elicits this allergic response followed by the changes produced by propagating virus. The lesion may then go through the same course as the primary vaccination or may exhibit an accelerated development of the lesion and its attendant erythema. Viral propagation is assumed to have occurred (and an immune response evoked) when the greatest area of skin involvement (erythema) occurs after the third day following revaccination. Revaccination is considered successful if a vesicular or pustular lesion is present or an area of definite palpable induration or congestion surrounding a central lesion, which may be a scar or ulcer, is present on examination 6-8 days after revaccination.3
INDICATIONS AND USAGE
Smallpox vaccine is indicated for active immunization against smallpox disease. The Advisory Committee on Immunization Practices (ACIP) recommends vaccination of laboratory workers who directly handle a) cultures or b) animals contaminated or infected with non-highly attenuated vaccinia virus, recombinant vaccinia viruses derived from non-highly attenuated vaccinia strains, or other Orthopoxviruses that infect humans (eg, monkeypox, cowpox, vaccinia and variola).2 The ACIP also recommends that vaccination can be considered for healthcare workers who have contact with clinical specimens, contaminated materials (eg, dressings), or patients receiving vaccinia or recombinant vaccinia viruses. Laboratory and other healthcare personnel who work with highly-attenuated poxvirus strains such as modified vaccinia Ankara (MVA), NYVAC (derived from the Copenhagen vaccinia strain), ALVAC (derived from canarypox virus), and TROVAC (derived from fowlpox virus) do not require routine vaccination.2
For those in the above special-risk categories, revaccination is recommended at appropriate intervals (every ten years).2
The Armed Forces continue to recommend use of smallpox vaccine for certain categories of personnel. See the most recent issue of Immunizations and Chemoprophylaxis, Departments of the Army, the Navy, the Air Force, and Transportation (Army Regulation 40-562, BUMEDINST 6230.15, Air Force Joint Instruction 48-110, CG COMDTINST M6230.4E)4 and Department of Defense Directive 6205.35 for current recommendations concerning use.
The judicious use of smallpox vaccine has been reported to have eradicated smallpox. At the World Health Assembly in May 1980, the World Health Organization (WHO) declared the world free of (naturally occurring) smallpox.6
As with any vaccine, smallpox vaccine may not protect all individuals receiving the vaccine.
Use of Smallpox Vaccine in Response to Bioterrorism:
Recommendations for use of smallpox vaccine in response to
bioterrorism are periodically updated by the Centers for Disease Control
and Prevention (CDC), and the most recent recommendations can be found at
http://www.cdc.gov/.
CONTRAINDICATIONS
Contraindications for Routine Non-Emergency Vaccine
Use
Primary vaccination AND revaccination with smallpox
vaccine are contraindicated:
- For any individuals who are allergic to any component of the vaccine, including polymyxin B sulfate, dihydrostreptomycin sulfate, chlortetracycline hydrochloride, and neomycin sulfate.
- Infants <12 months of age. The ACIP advises against non-emergency use of smallpox vaccine in children <18 years of age.2
- For individuals of any age with eczema or past history of eczema or for those whose household contacts have eczema, other acute, chronic, or exfoliative skin conditions, (eg, atopic dermatitis, wounds, burns, impetigo, or Varicella zoster) and for siblings or other household contacts of such individuals.2
- For persons of any age receiving therapy with systemic corticosteroids at certain doses (eg, >2 mg/kg body weight or >20 mg/day of prednisone for >2 weeks),2 or immunosuppressive drugs (eg, alkylating agents, antimetabolites), or radiation. Household contacts of such persons should not be vaccinated.
- For individuals with congenital or acquired deficiencies of the immune system, including individuals infected with the human immunodeficiency virus (HIV). Household contacts of such persons should not be vaccinated.
- For individuals with immunosuppression (eg, leukemia, lymphomas of any type, generalized malignancy, solid organ transplantation, hematopoietic stem cell transplantation, cellular or humoral immunity disorders, agammaglobulinemia, or other malignant neoplasms affecting the bone marrow or lymphatic systems), or household contacts of such individuals.2
- During pregnancy, suspected pregnancy, or to household contacts of pregnant women.
Contraindications for Smallpox Emergency
There are
no absolute contraindications regarding vaccination of a person with a
high-risk exposure to smallpox.2 Persons at greatest risk for
experiencing serious vaccination complications are often those at greatest
risk for death from smallpox. If a relative contraindication to
vaccination exists, the risk for experiencing serious vaccination
complications must be weighed against the risks for experiencing a
potentially fatal smallpox infection.2
PRECAUTIONS
General
The vial stopper contains dry natural
rubber that may cause hypersensitivity reactions when handled by, or when
the product is administered to, persons with known or possible latex
sensitivity.
After completion of the multiple-puncture vaccination, blot off any vaccine remaining on skin at vaccination site with clean, dry gauze or cotton.
The vaccine vial, its stopper, the needle to release the vacuum, the diluent syringe, the vented needle used for reconstitution, the bifurcated needle used for administration, and any gauze or cotton that came in contact with the vaccine should be burned, boiled, or autoclaved before disposal.
Individuals susceptible to adverse effects of vaccinia virus, eg, those with eczema, immunodeficiency states, including HIV infection, should be identified and measures taken to avoid contact with persons with active vaccination lesions. Contact spread of vaccinia from recently vaccinated military personnel has been reported7,8 (see ADVERSE REACTIONS).
Prevention of contact transmission of vaccinia
Vaccinia
virus may be cultured from the site of primary vaccination beginning at
the time of development of a papule (2 to 5 days after vaccination) until
the scab separates from the skin lesion (14 to 21 days after vaccination).
During this time, care must be taken to prevent spread of the virus to
another area of the body or to another person. The vaccination site may be
left uncovered or can be covered with a porous bandage, such as gauze,
until the scab has separated and the underlying skin has healed. An
occlusive bandage should not be routinely used. If a bandage is used to
cover the vaccination site, it should be changed frequently (ie, every 1-2
days) to prevent maceration of the vaccination site secondary to fluid
accumulation. No salves or ointments should be used on the vaccination
site. Contaminated bandages should be placed in sealed plastic bags before
disposal in the trash. Clothing or other cloth materials that have had
contact with the site can be decontaminated with routine laundering in hot
water with bleach.2 The vaccination site should be kept dry,
although normal bathing can continue.2
Recently vaccinated healthcare workers should avoid contact with patients, particularly those with immunodeficiencies, until the scab has separated from the skin at the vaccination site. However, if continued contact with patients is essential and unavoidable, they may continue to have contact with patients, including those with immunodeficiencies, as long as the vaccination site is well covered and good hand-washing technique is maintained by the vaccinee. In this setting, a more occlusive dressing may be required. Semipermeable polyurethane dressings (eg, Opsite®) are effective barriers to vaccinia and recombinant vaccinia viruses. However, exudate may accumulate beneath the dressing, and care must be taken to prevent viral contamination when the dressing is removed. In addition, accumulation of fluid beneath the dressing may increase the maceration of the vaccination site. Accumulation of exudate may be decreased by first covering the vaccination with dry gauze, then applying the dressing over the gauze. The dressing should also be changed at least once a day.
The most important measure to prevent inadvertent implantation and contact transmission from vaccinia vaccination is thorough hand washing after changing the bandage or after any other contact with the vaccination site.
Simultaneous administration with other live-virus
vaccines
There are no data evaluating the simultaneous
administration of smallpox vaccine with other live-virus vaccines.
PREGNANCY
Pregnancy Category C
Animal
reproduction studies have not been conducted with smallpox vaccine.
Smallpox vaccine should not be given to pregnant women in routine,
non-emergency conditions. For emergency conditions, see
CONTRAINDICATIONS - Contraindications for Smallpox Emergency and
INDICATIONS AND USAGE - Use of Smallpox Vaccine in Response to
Bioterrorism. On rare occasions, almost always after primary
vaccination, vaccinia virus has been reported to cause fetal infection.
Fetal vaccinia usually results in stillbirth or death of the infant
shortly after delivery. Vaccinia vaccine is not known to cause congenital
malformations.2
Nursing Mothers
It is not known whether vaccine
antigens or antibodies are excreted in human milk. This vaccine is not
recommended for use in a nursing mother in non-emergency conditions. For
use in emergency conditions, see CONTRAINDICATIONS -
Contraindications for Smallpox Emergency.
Pediatric Use
Before the eradication of smallpox
disease, smallpox vaccination was administered routinely during childhood.
The vaccine is considered safe and effective in children. However,
smallpox vaccine is not recommended for use in non-emergency situations
and is contraindicated for infants <12 months in non-emergency
situations.
Geriatric Use
There are no published data to
support the use of this vaccine in geriatric populations. This vaccine is
not recommended for use in geriatric populations in non-emergency
conditions. For use in emergency conditions, see CONTRAINDICATIONS
- Contraindications for Smallpox Emergency.
ADVERSE REACTIONS
A fever is common after vaccinia
vaccination is administered. Up to 70% of children have one or more days
of temperature >100°F from 4 to 14 days after primary
vaccination, and 15% to 20% have temperatures of >102°F. After
revaccination, 35% of children develop temperatures of >100°F,
and 5% have temperatures of >102°F. Fever is less common in
adults than children after vaccination or revaccination.2
Generalized rashes (erythematous, urticarial, nonspecific) and secondary pyogenic infections at the site of vaccine applications may occur. Rarely bullous erythema multiforme (Stevens-Johnson syndrome) occurs.2
Inadvertent inoculation at other sites is the most frequent complication of vaccinia vaccination, usually resulting from autoinoculation of the vaccine virus transferred from the site of vaccination. The most common sites involved are the face, eyelid, nose, mouth, genitalia, and rectum. Accidental infection (autoinoculation) of the eye may result in blindness. Generalized vaccinia among persons without underlying illnesses is characterized by a vesicular rash of varying extent. The rash is generally self-limited and requires little or no therapy except among patients whose conditions appear to be toxic or who have serious underlying illnesses.2 Contact spread of vaccinia from recently vaccinated military personnel has been reported (see CONTRAINDICATIONS).7,8,9
More severe complications that may follow either primary vaccination or revaccination include: postvaccinial encephalitis, encephalomyelitis, encephalopathy, progressive vaccinia (vaccinia necrosum), and eczema vaccinatum. Such complications may result in severe disability, permanent neurological sequelae, and/or death.10,11 Although a rare event, approximately 1 death per million primary vaccinations and 1 death per 4 million revaccinations have occurred after vaccinia vaccination. Death is most often the result of postvaccinial encephalitis or progressive vaccinia.2, 12 , Death has also been reported in unvaccinated contacts of individuals who have been vaccinated.12
Estimates of the risks of occurrence of complications after primary vaccination and revaccination are as follows13:
Rates of reported complications* associated with vaccinia vaccinations (cases/million vaccinations)†
| Age (yrs) and status | Inadvertent inoculation§ | Generalized vaccinia | Eczema vaccinatum | Progressive vaccinia¶ | Postvaccinial encephalitis | Death10,# | Total** |
| Primary vaccination | |||||||
| <1 | 507.0 | 394.4 | 14.1 | --†† | 42.3 | 5 | 1549.3 |
| 1-4 | 577.3 | 233.4 | 44.2 | 3.2 | 9.5 | 0.5 | 1261.8 |
| 5-19 | 371.2 | 139.7 | 34.9 | --†† | 8.7 | 0.5 | 855.9 |
| >20 | 606.1 | 212.1 | 30.3 | --†† | --†† | unknown | 1515.2 |
| Overall rates† | 529.2 | 241.5 | 38.5 | 1.5 | 12.3 | -- | 1253.8 |
| Revaccination | |||||||
| <1 | --†† | --†† | --†† | --†† | --†† | -- | --†† |
| 1-4 | 109.1 | --†† | --†† | --†† | --†† | -- | 200.0 |
| 5-19 | 47.7 | 0.9 | 2.0 | --†† | --†† | -- | 85.5 |
| >20 | 25.0 | 9.1 | 4.5 | 6.8 | 4.5 | -- | 113.6 |
| Overall rates§§ | 42.1 | 9.0 | 3.0 | 3.0 | 2.0 | -- | 108.2 |
* See text for descriptions of complications.
§ Referenced as accidental implantation.
¶ Referenced as vaccinia necrosum.
# Death from all complications.10
** Rates of overall complications by age group include complications not provided in this table, including severe local reactions, bacterial superinfection of the vaccination site, and erythema multiforme.
†† No instances of this complication were identified during the 1968 10-state survey.
§§ Overall rates for each complication include persons of unknown age.
The risk of complications associated with revaccination is low. Complications have occurred, especially in patients with underlying diseases or in patients receiving therapy which impairs immunologic competence, or in subjects who have not been vaccinated for many years. Subjects who have not been vaccinated for many years may respond as primary vaccinees as regards both the local and systemic reaction to vaccine administration and risk of occurrence of the above-mentioned serious complications.2
The Centers for Disease Control and Prevention (CDC) can assist physicians in the diagnosis and management of patients with suspected complications of vaccinia (smallpox) vaccination. Vaccinia Immune Globulin (VIG) is indicated for certain complications of smallpox vaccination. Several antiviral compounds have been shown to have activity against vaccinia virus or other orthopoxviruses in vitro and in animal models. However, insufficient information exists on which to base recommendations for any antiviral compound to treat postvaccination complications or Orthopoxvirus infections, including smallpox.2 If VIG is needed or additional information is required, physicians should contact the CDC at (404) 639-3670, Monday through Friday 8AM to 4:30 PM Eastern Standard Time; at other times call (404) 639-2888. The United States Department of Health and Human Services (DHHS) has established the Vaccine Adverse Event Reporting System (VAERS) to accept all reports of suspected adverse events of any vaccine. The VAERS toll-free number for VAERS forms and information is (800-822-7967).
DOSAGE AND ADMINISTRATION
Read all directions
completely before beginning reconstitution and administration. When
reconstituting and administering the vaccine, use protective gloves and
aseptic technique.
Directions for Reconstitution:
Note: The healthcare
provider must have available a sterile 21 gauge or smaller needle to
release the vacuum in the vials prior to adding diluent. This needle must
only be used to release the vacuum. The needle to release the vacuum is
NOT included in the kit.
- Lift up tab of aluminum seal on vaccine vial. DO NOT BREAK OFF OR
TEAR DOWN TAB.
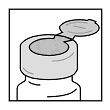
- Wipe off vial stopper with an alcohol sponge and allow to dry.
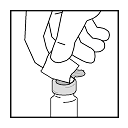
- Place vaccine vial upright on a hard, flat surface. Insert a sterile
21 gauge or smaller needle into the rubber stopper to release the vacuum
from the vaccine vial. Discard the needle in biohazard waste container.
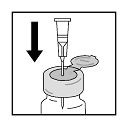
- To reduce viscosity of cold diluent, warm by holding diluent-cartridge in palm of hand for a minute or so.
- Peel open the vented needle package (provided with the kit) and aseptically remove the vented needle.
- Remove rubber cover from end of the diluent syringe.
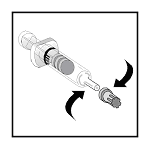
- With a twisting motion, aseptically attach the vented needle to the
hub of the diluent syringe.
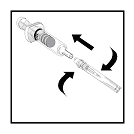
- Remove protective cover from the vented needle and expel the air
from the diluent syringe.
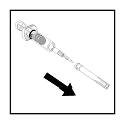
- Aseptically insert the needle through the rubber stopper into the
vaccine vial up to the first hub.
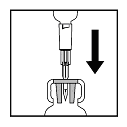
- Depress the plunger to ensure the entire volume of diluent is
delivered into the vial.
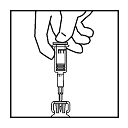
- Withdraw diluent syringe/vented needle and discard in biohazard waste container.
- Allow vaccine vial to stand undisturbed for 3 to 5 minutes. Then if necessary, swirl vial gently to effect complete reconstitution.
- Record date of reconstitution.
- Store reconstituted vaccine at 2° to 8°C (36° to 46°F) when not in actual use. The vaccine may be stored for no more than 15 days after reconstitution.
Sites of Vaccination:
The skin over the insertion
of the deltoid muscle or the posterior aspect of the arm over the triceps
muscle is the preferred site for smallpox vaccination.
Method of Vaccination:
USE 2 OR 3 NEEDLE PUNCTURES
FOR PRIMARY VACCINATION; 15 FOR REVACCINATION.
Reconstituted vials should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use vaccine if particulate matter or discoloration is present.
- First pull down the “tear off” tab from the aluminum seal of the
vaccine vial.
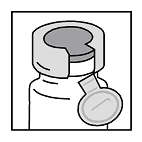
- Remove entire aluminum seal from the vaccine vial. Then remove
rubber stopper from vaccine vial and aseptically retain stopper (set
aside inverted) for subsequent reuse.
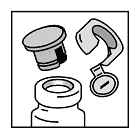
- The skin over the insertion of the deltoid muscle or the posterior aspect of the arm over the triceps muscle is the preferred site for smallpox vaccination. If alcohol is used to clean the site, the skin must be allowed to dry thoroughly to prevent inactivation of the vaccine by the alcohol.
- Tear off a packette containing a single, sterile bifurcated vaccinating needle.
- Peel back the packaging approximately halfway exposing the butt-end
of needle.
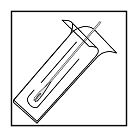
- Hold butt-end of needle and gently pull bifurcated point end free of packaging.
- Carefully dip bifurcated end of needle into vaccine. Visually
confirm that the needle picks up a drop of vaccine in the space between
the two tips.
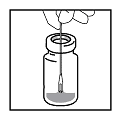
- Deposit the drop of vaccine onto clean, DRY site previously prepared for vaccination. Do not redip needle into vaccine if needle has touched skin.
- With the same needle, and using multiple-puncture technique, vaccinate through drop of vaccine. Holding the bifurcated needle perpendicular to the skin, punctures are rapidly made with strokes vigorous enough to allow a trace of blood to appear after 15-20 seconds. Two or 3 punctures are recommended for primary vaccination; 15 punctures for revaccination. Any remaining vaccine should be wiped off with dry sterile gauze and the gauze disposed of in a biohazard waste container.
- Discard needle in a biohazard waste container.
- Repeat Steps 3 through 10 for each individual to be vaccinated utilizing a new bifurcated needle for each individual vaccinated.
- If vaccine is to be stored for subsequent use, re-stopper the vial with the rubber stopper and store at 2° to 8°C (36° to 46°F). The vaccine may be stored for no more than 15 days after reconstitution.
- When next needed, remove vial from refrigerator, gently swirl suspension to ensure resuspension, and then carefully take off stopper-cap.
- Repeat Steps 3 through 10.
- If vaccine is to be restored for subsequent use, replace stopper-cap and store at 2° to 8°C (36° to 46°F). The vaccine may be stored for no more than 15 days after reconstitution.
Interpretation of Responses:
The vaccination site
should be inspected 6 to 8 days after vaccination. Two types of responses
have been defined by the World Health Organization (WHO) Expert Committee
on Smallpox.3 They are: 1) major reaction, indicating that
virus replication has taken place and vaccination was successful; or 2)
equivocal reaction, indicating a possible consequence of immunity capable
of suppressing viral multiplication or allergic reactions to an inactive
vaccine with production of immunity.
Major Reaction
Major reaction is defined as a vesicular or
pustular lesion or an area of definite palpable induration or congestion
surrounding a central lesion that might be a crust or an ulcer. The
inoculation site becomes reddened and pruritic 3-4 days after vaccination.
A vesicle surrounded by a red areola then forms, which becomes umbilicated
and then pustular the 7th to 11th day after vaccination, and the pustule
begins to dry, the redness subsides, and the lesion usually becomes
crusted between the 14th and 21st days. By the end of approximately the
third week, the scab falls off, leaving a permanent scar, which at first
is pink in color but eventually becomes flesh-colored (see
CLINICAL PHARMACOLOGY).2
Primary vaccination may be accompanied by fever, regional lymphadenopathy, and malaise persisting for a few days.
Revaccination is considered successful if a vesicular or pustular lesion is present or an area of definite palpable induration or congestion surrounding a central lesion, which may be a scar or ulcer, is present on examination 6-8 days after revaccination.3 Major reactions, especially when there has been an interval of many years since the last successful vaccination, may be accompanied by fever, regional lymphadenopathy, and malaise persisting for a few days.
Equivocal Reaction
Equivocal reactions are defined as all
responses other than major reactions.3 If an equivocal reaction
is observed, vaccination procedures should be checked and vaccination
repeated with vaccine from another vial or vaccine lot, if available. If a
repeat vaccination by using vaccine from another vial or vaccine lot fails
to produce a major reaction, healthcare providers should consult CDC or
their state or local health department before giving another
vaccination.2
HOW SUPPLIED
Combination package of 1 vial of Dried Smallpox Vaccine, 1 Diluent
syringe (0.25 mL), 1 vented needle, 100 individually wrapped bifurcated
needles (20 strips, 5 needles per strip). Note: The healthcare provider
must have available a sterile 21 gauge or smaller needle to release the
vacuum in the vials prior to adding diluent. The needle to release the
vacuum is NOT included in the kit.
NDC# 0008-0348-08
STORAGE
Store un-reconstituted Dryvax® in the refrigerator (2° to
8°C, 36° to 46°F). DO NOT FREEZE.
RECONSTITUTED Dryvax® may
be used for 15 days if stored at 2° to 8°C (36° to 46°F) when not in
actual use. At time of reconstitution, record date. Dryvax®
should not be used after the expiration date regardless of whether it is
in the dry or reconstituted form.
REFERENCES
- Cherry JD, McIntosh K, Connor JD, et al. Primary percutaneous vaccination. J Infect Dis. 1977;135:145-154
- Vaccinia (Smallpox) Vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2001. MMWR. 2001;50(RR10):1-25.
- WHO Expert Committee on Smallpox. First report. Geneva: World Health Organization;1964. World Health Organization Technical Report Series, No. 283.
- Immunization Requirements and Procedures, Department of the Army, the Navy, the Air Force, and Transportation. 1995. Available from http://www.e-publishing.af.mil/pubfiles/af/48/afji48-110/afji48-110.pdf . Accessed August 8, 2002
- Department of Defense Directive. Number 6205.3. DoD Immunization Program for Biological Warfare Defense. 1993. Available from http://www.dtic.mil/whs/directives/corres/pdf/d62053_112693/d62053p.pdf. Accessed August 8, 2002.
- WHO Policy System: The Thirty-third World Health Assembly. Declaration of global eradication of smallpox, Geneva, May 23, 1980.
- Contact spread of vaccinia from a recently vaccinated Marine - Louisiana. MMWR. 1984;33(3):37-38.
- Contact spread of vaccinia from a National Guard vaccinee - Wisconsin. MMWR. 1985;34(13):182-183.
- Vaccinia outbreak - Nevada. MMWR. 1983;32(31):403-404.
- Lane J, Millar J. Risks of smallpox vaccination complications in the United States. Am J Epidemiol. 1971;93:238-240.
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its Eradication. World Health Organization. Geneva, 1988. Available at http://www.who.int/emc/diseases/smallpox/Smallpoxeradication.html. . Accessed August 8, 2002.
- Lane J, Ruben F, Neff J, Millar J. Complications of smallpox vaccination, 1968: national surveillance in the United States. N Engl J M. 1969;281(22):1201-1208.
- Lane J, Ruben F, Neff J, Millar J. Complications of smallpox vaccination, 1968: results of ten statewide surveys. J Infect Dis. 1970;122 (4):303-309.
Manufactured by:
| ® |
Wyeth Laboratories A Wyeth-Ayerst Company Marietta, PA 17547 USA |
U.S. Govt. Lic. No. 3
Last Updated: 11/6/2002