Home |
Climate Change Project |
Table of Contents |
Courses | Search |
Final Report of the Task Force on Genetic TestingSeptember 1997
EDITORS: Neil A. Holtzman, M.D., MPH
Michael S. Watson, Ph.D.The Task Force was created by the National Institutes of Health-Department of Energy Working Group on Ethical, Legal, and Social Implications of Human Genome Research.
AcknowledgmentsThe work of the Task Force was accomplished with the help of many others. Joshua Brown served as staff attorney for the Task Force from April 1995 to May 1996. Mr. Brown prepared very helpful briefing materials and presentations for the Task Force on laws and regulations relating to genetic testing, coordinated Task Force committee meetings, and assisted in the formulation of Task Force principles and recommendations. After Mr. Brown's departure, Emily Koscianski and Andrew Siegel continued legislative and regulatory analysis and the drafting of proposed recommendations on a part-time basis until April 1997. Dr. Siegel, a Greenwall Fellow, was particularly helpful in framing issues related to institutional review boards. Jodi Goldstein, Taria Herz, Katherine Lester, Amanda Merwin, and Michele Schoonmaker, at the time graduate students at the Johns Hopkins School of Hygiene and Public Health (Goldstein and Lester in the joint Hopkins-Georgetown University program in law, ethics, and health), also prepared very useful background papers. Jane Fullarton's familiarity with the Department of Health and Human Services proved extremely valuable in preparing the Task Force's proposed recommendations. At the Genetics and Public Policy Studies unit at the Johns Hopkins Medical Institutions, where much of the work was done, Sharon Ennis coordinated administrative tasks and Robin Wingfield-Street maintained mailing lists and assembled briefing materials for the Task Force. Over the more than 2-year life of the Task Force, Tascon was responsible for scheduling and arranging meetings (including travel of Task Force members), final mailings, publication of the report, and overall administration. We wish to thank, particularly, Rose Salton, Cindy Elliott-Amadon, and Nancy Shapiro. The appendices to the report were edited by Alice Lium, and the main body of the report by Barbara Cobb. Cindy James, a graduate student in genetic counseling and human genetics, checked references and analyzed trends in genetic discoveries and resources. Support for the Task Force was generously provided by the National Human Genome Research Institute (NHGRI). The Task Force is grateful for the personal interest Francis Collins, Director of NHGRI, took in its work and for his very helpful input. Finally, every voting and liaison member of the Task Force played an active role in the development of the Task Force's principles and recommendations, and the preparation of the report. Patricia Murphy generously agreed to help in the writing of Chapter 3. Carlyn Collins of the Centers for Disease Control and Prevention, Kate Kremann of the Health Care Financing Administration, and Freda Yoder of the Food and Drug Administration attended many Task Force meetings, reviewed briefing materials and drafts, and made many helpful suggestions. Voting Members: Neil A. Holtzman, M.D., M.P.H., Chair ELSI Working Group Michael S. Watson, Ph.D., FACMG, Co-Chair American College of Medical Genetics Patricia A. Barr National Breast Cancer Coalition David R. Cox, M.D., Ph.D. ELSI Working Group Jessica G. Davis, M.D. Council of Regional Networks for Genetic Services Stephen I. Goodman, M.D., M.Sc. American Society of Human Genetics Wayne W. Grody, M.D., Ph.D. College of American Pathologists Arthur L. Levin, M.D. Alliance for Managed Competition J. Alexander Lowden, M.D., Ph.D. Health Insurance Association of America Patricia D. Murphy, Ph.D., FACMG OncorMed Patricia J. Numann, M.D. American Medical Association Victoria O. Odesina, R.N., Sc.M., M.S. Alliance of Genetic Support Groups Nancy Press, Ph.D. ELSI Working Group Katherine A. Schneider, M.P.H. National Society of Genetic Counselors David B. Singer Biotechnology Industry Organization (BIO) Elliott Hillback was BIO alternate representative when Mr. Singer could not attend. Government Liaison (Non-voting) Members: Steven Gutman, M.D. Food and Drug Administration Muin J. Khoury, M.D., Ph.D. Centers for Disease Control and Prevention David Lanier, M.D. Agency for Health Care Policy and Research Peter Bouxsein, M.D. represented the Agency until September 1996. Linda R. Lebovic Health Care Financing Administration Jane S. Lin-Fu, M.D. Health Resources and Services Administration The work of the Task Force was supported by the National Institutes of Health.
EXECUTIVE SUMMARY
The rapid pace of discovery of genetic factors in disease has improved our ability to predict risks of disease in asymptomatic individuals. We have learned how to prevent the manifestations of a few of these diseases and treat some others. Gene therapy is being actively investigated.
Despite remarkable progress much remains unknown about the risks and benefits of genetic testing.
· No effective interventions are yet available to improve the outcome of most inherited diseases.
· Negative (normal) test results might not rule out future occurrence of disease.
· Positive test results might not mean the disease will inevitably develop.
It is primarily in the context of their unknown potential risks and benefits that the Task Force considers genetic testing.
Origin and Work of the Task Force
The Task Force was created by the National Institutes of Health (NIH)-Department of Energy (DOE) Working Group on Ethical, Legal, and Social Implications (ELSI) of Human Genome Research to review genetic testing in the United States and make recommendations to ensure the development of safe and effective genetic tests. The Task Force has defined safety and effectiveness to encompass not only the validity and utility of genetic tests, but their delivery in laboratories of assured quality, and their appropriate use by health care providers and consumers.
The Working Group invited organizations with a stake in genetic testing to submit nominations from which it selected members of the Task Force. In addition, the Working Group invited five agencies in the Department of Health and Human Services (HHS) to send nonvoting liaison members to the Task Force. Principles and recommendations of the Task Force appear in bold-faced type.
Definition of Genetic Tests
Genetic test--The analysis of human DNA, RNA, chromosomes, proteins, and certain metabolites in order to detect heritable disease-related genotypes, mutations, phenotypes, or karyotypes for clinical purposes. Such purposes include predicting risk of disease, identifying carriers, and establishing prenatal and clinical diagnosis or prognosis. Prenatal, newborn and carrier screening, as well as testing in high risk families, are included. Tests for metabolites are covered only when they are undertaken with high probability that an excess or deficiency of the metabolite indicates the presence of heritable mutations in single genes. Tests conducted purely for research are excluded from the definition, as are tests for somatic (as opposed to heritable) mutations, and testing for forensic purposes.
The Task Force is primarily concerned about predictive uses of genetic tests performed in healthy or apparently healthy people. Predictive test results do not necessarily mean that the disease will inevitably occur or remain absent; they replace the individual's prior risks based on population data or family history with risks based on genotype. Some, but not all, predictive genetic testing falls under the rubric "genetic screening," a search in a population for persons possessing certain genotypes.
The Need for Recommendations
For the most part, genetic testing in the United States has developed successfully, providing options for avoiding, preventing, and treating inherited disorders. However, problems arise as a result of current practices.
· Sometimes, genetic tests are introduced before they have been demonstrated to be safe, effective, and useful (see chapter 2 and appendices 5 and 6).
· There is no assurance that every laboratory performing genetic tests for clinical purposes meets high standards (see chapter 3).
· Often, the informational materials distributed by academic and commercial genetic testing laboratories do not provide sufficient information to fill in the gaps in providers' and patients' understanding of genetic tests (see appendix 4).
· In the next few years, a greater burden for offering genetic testing will fall on providers who have little formal training or experience in genetics.
In this report, the Task Force does not recommend policies for specific tests but
suggests a framework for ensuring that new tests meet criteria for safety and effectiveness before they are unconditionally released, thereby reducing the likelihood of premature clinical use. The focus of the Task Force on potential problems in no way is intended to detract from the benefits of genetic testing. Its overriding goal is to recommend policies that will reduce the likelihood of damaging effects so the benefits of testing can be fully realized undiluted by harm.Need for an Advisory Committee on Genetic Testing
The Task Force calls on the Secretary of Health and Human Services (HHS) to establish an advisory committee on genetic testing in the Office of the Secretary. Members of the committee should represent the stakeholders in genetic testing, including professional societies (general medicine, genetics, pathology, genetic counseling), the biotechnology industry, consumers, and insurers, as well as other interested parties. The various HHS agencies with activities related to the development and delivery of genetic tests should send nonvoting representatives to the advisory committee, which can also coordinate the relevant activities of these agencies and private organizations. The Task Force leaves it to the Secretary to determine the relationship of this advisory committee to others that may be created in the broader area of genetics and public policy, of which genetic testing is only one part.
The committee would advise the Secretary on implementation of recommendations made by the Task Force in this report to ensure that (a) the introduction of new genetic tests into clinical use is based on evidence of their analytical and clinical validity, and utility to those tested; (b) all stages of the genetic testing process in clinical laboratories meet quality standards; (c) health providers who offer and order genetic tests have sufficient competence in genetics and genetic testing to protect the well-being of their patients; and (d) there be continued and expanded availability of tests for rare genetic diseases.
The Task Force recognizes the widely inclusive nature of genetic tests. It is therefore essential that the advisory committee recommend policies for the Secretary's consideration by which agencies and organizations implementing recommendations can determine those genetic tests that need stringent scrutiny. Stringent scrutiny is indicated when a test has the ability to predict future inherited disease in healthy or apparently healthy people, is likely to be used for that purpose, and when no confirmatory test is available. The advisory committee or its designate should define additional indications.
In order to carry out its functions, the advisory committee should have its own staff and budget.
The Task Force further recommends that the Secretary review the accomplishments of the advisory committee on genetic testing after 2 full years of operation and determine whether it should continue to operate.
Overarching Principles
In making recommendations on safety and effectiveness, the Task Force concentrated on test validity and utility, laboratory quality, and provider competence. It recognizes, however, that other issues impinge on testing, and problems may arise from testing. Regarding these issues, the Task Force endorses the following principles.
Informed Consent. The Task Force strongly advocates written informed consent. The failure of the Task Force to comment on informed consent for other uses does not imply that it should not be obtained.
Test Development. Informed consent for any validation study must be obtained whenever the specimen can be linked to the subject from which it came.
Testing in Clinical Practice. (1) It is unacceptable to coerce or intimidate individuals or families regarding their decision about predictive genetic testing. Respect for personal autonomy is paramount. People being offered testing must understand that testing is voluntary. Their informed consent should be obtained. Whatever decision they make, their care should not be jeopardized.
(2) Prior to the initiation of predictive testing in clinical practice, health care providers must describe the features of the genetic test, including potential consequences, to potential test recipients.
Newborn Screening. (1) If informed consent is waived for a newborn screening test, the analytical and clinical validity and clinical utility of the test must be established, and parents must be provided with sufficient information to understand the reasons for screening. By clinical utility, the Task Force means that interventions to improve the outcome of the infant identified by screening have been proven to be safe and effective.
(2) For those disorders for which newborn screening is available but the tests have not been validated or shown to have clinical utility, written parental consent is required prior to testing.
Prenatal and Carrier Testing. Respect for an individual's/couples' beliefs and values concerning tests undertaken for assisting reproductive decisions is of paramount importance and can best be maintained by a nondirective stance. One way of ensuring that a non-directive stance is taken and that parents' decisions are autonomous, is through requiring informed consent.
Testing of Children. Genetic testing of children for adult onset diseases should not be undertaken unless direct medical benefit will accrue to the child and this benefit would be lost by waiting until the child has reached adulthood.
Confidentiality. Protecting the confidentiality of information is essential for all uses of genetic tests. (1) Results should be released only to those individuals for whom the test recipient has given consent for information release. Means of transmitting information should be chosen to minimize the likelihood that results will become available to unauthorized persons or organizations. Under no circumstances should results with identifiers be provided to any outside parties, including employers, insurers, or government agencies, without the test recipient's written consent.
(2) Health care providers have an obligation to the person being tested not to inform other family members without the permission of the person tested, except in extreme circumstances.
Discrimination. No individual should be subjected to unfair discrimination by a third party on the basis of having had a genetic test or receiving an abnormal genetic test result. Third parties include insurers, employers, and educational and other institutions that routinely inquire about the health of applicants for services or positions.
Consumer Involvement in Policy Making. Although other stakeholders are concerned about protecting consumers, they cannot always provide the perspective brought by consumers themselves, the end users of genetic testing. Consumers should be involved in policy (but not necessarily in technical) decisions regarding the adoption, introduction, and use of new, predictive genetic tests.
ENSURING THE SAFETY AND EFFECTIVENESS OF NEW GENETIC TESTS
Providers and consumers cannot make a fully-informed decision about whether or not to use genetic tests unless their benefits and risks have been assessed. Although extensive use has eventually proved most tests to be of benefit, a few eventually proved unhelpful and were discarded. In the meantime, people were wrongly classified as at-risk and subjected to treatments that, in their case, proved unnecessary or sometimes harmful. Others, who could have benefited from treatment were classified as "normal" and denied treatment. The Task Force strongly recommends that the following criteria be satisfied.
(1) The genotypes to be detected by a genetic test must be shown by scientifically valid methods to be associated with the occurrence of a disease. The observations must be independently replicated and subject to peer review.
(2) Analytical sensitivity and specificity of a genetic test must be determined before it is made available in clinical practice.
(3) Data to establish the clinical validity of genetic tests
(clinical sensitivity, specificity, and predictive value) must be collected under investigative protocols. In clinical validation, the study sample must be drawn from a group of subjects representative of the population for whom the test is intended. Formal validation for each intended use of a genetic test is needed.(4)
Before a genetic test can be generally accepted in clinical practice, data must be collected to demonstrate the benefits and risks that accrue from both positive and negative results.Ensuring Compliance with Criteria for Safety and Effectiveness
Because of the length of time it can take to establish the appropriateness of a test for clinical use, it is all the more important to ensure the collection of data on safety and effectiveness in the course of test development. At present, no government policy requires the collection of data on clinical validity and utility for all predictive genetic tests under development.
Considering the structures for external review of research in the U.S. today, the Task Force is of the opinion that institutional review boards (IRBs) are the most appropriate organizations to consider whether the scientific merit of protocols for the development of genetic tests warrants the risk to subjects participating in the research.
Protocols for the development of genetic tests that can be used predictively must receive the approval of an institutional review board (IRB) when subject identifiers are retained and when the intention is to make the test readily available for clinical use, i.e., to market the test. IRB review should consider the adequacy of the protocol for: (a) the protection of human subjects involved in the study, and (b) the collection of data on analytic and clinical validity, and data on the test's utility for individuals who are tested.
Tests under development must be conducted in laboratories certified under the Clinical Laboratory Improvement Amendments (CLIA) if the results will be reported to patients or their providers.
Health department laboratories or other public agencies developing new genetic tests that satisfy these conditions must also submit protocols to properly constituted IRBs.
The Task Force recommends that the Office of Protection of Human Subjects from Research Risks (OPRR) develop guidelines to assist IRBs in reviewing genetic testing protocols. The proposed Secretary's Advisory Committee should work with OPRR to accomplish this task. In developing guidelines for IRBs, OPRR should focus first on tests under development that require stringent scrutiny. The proposed Secretary's Advisory Committee or its designate, in cooperation with OPRR, should establish criteria for stringent scrutiny.
Conflict of Interest. The Task Force recommends strenuous efforts by all IRBs (commercial and academic) to avoid conflicts of interest, or the appearance of conflicts of interest, when reviewing specific protocols for genetic testing. OPRR should consider more stringent standards for all types of IRBs for avoiding conflict of interest situations.
Enforcement
. Testing organizations should comply voluntarily with obtaining IRB approval of genetic test protocols. Other options the Task Force considered for enforcing the requirement for IRB approval included that: (1) the FDA use its authority to require all test developers to submit protocols to IRBs, (2) third-party payers refuse to reimburse for a genetic test unless the developer can show that it conducted validation/utility studies under an IRB-approved protocol, (3) clinical laboratory surveyors (see chapter 3) confirm that laboratories have received IRB approval of the new genetic tests that they developed, and (4) Congress enact legislation requiring submission of all research protocols, regardless of support, to an IRB.Data Collection. To expedite data collection, collaborative efforts will often be needed. OPRR, with input from the proposed Secretary's Advisory Committee on genetic testing, should streamline the requirements for IRB review of multicenter collaborative protocols for genetic test development in order to reduce costs and get the studies quickly underway.
The Task Force calls on Federal agencies, particularly NIH and the Centers for Disease Control and Prevention (CDC) to support consortia and other collaborative efforts to facilitate collection of data on the safety and effectiveness of new genetic tests. CDC should play a coordinating role in data gathering and should be allocated sufficient funds for this purpose. In sharing or pooling of data, confidentiality of the subject source of the data must be strictly maintained.
The Need for Post-market Surveillance. The Task Force recognizes that assessing the validity and utility of some genetic tests will take a long time. When preliminary data indicate a test is likely to have validity and utility, the test should be approved for marketing (see below) but developers must continue to collect data until more definitive answers are obtained. Options for encouraging collection of the requisite data include the following:
(1) Voluntary collection of data by developers after their tests enter clinical use.
(2) Reimbursement for, or coverage of, tests by third party payers during investigative stages in which data are being collected.
(3) Conditional premarket approval by the FDA of genetic test kits. In return for conditional approval, developers could include a profit markup in the price while they continue to collect data.
Evidence-based Entry of New Genetic Tests into Clinical Practice
Test developers must submit their validation and clinical utility data to internal as well as independent external review.
In addition, test developers should provide information to professional organizations in order to permit informed decisions about routine use. The Task Force recognizes that not all new genetic tests are in need of such review. The proposed Secretary's Advisory Committee should suggest criteria for external review, and recommend means of ensuring that review of tests requiring stringent scrutiny will take place. To accomplish the latter, the cooperation of various government and nongovernment groups to conduct reviews must be secured, as well as funds to support the reviews. A wide range of stakeholders should participate in reviews.Local Review. The Task Force strongly suggests that any organization in which tests are developed conduct a structured review of the analytic and clinical validity and utility of new genetic tests before marketing them or otherwise making them available for clinical use. This structured review should be conducted by those not actually involved in developing the test and collecting the data. Some medical centers have standing committees that review tests proposed to be offered in the institution's clinical laboratories that could serve this function. For commercial organizations, a unit within the company, but independent of the laboratory that is actually developing the test, should review the data.
National Review
. Current legal requirements that genetic tests be reviewed prior to their clinical use apply only to tests marketed as kits, which require premarket approval by FDA. To improve FDA perspectives on genetic testing and related issues, the Task Force recommends that FDA bring together consultants on genetic testing either from existing panels or by constructing a new panel to provide guidance to FDA on genetic testing devices with single or multiple intended uses.Although no other legally-required mechanisms currently exist, other reviews can have a profound influence on providers' decisions to use, or not use, new medical technologies. Examples are: statements of professional societies, consensus development panels, and ratings by the U.S. Preventive Services Task Force. The decision of health insurers on whether a specific genetic test will be included in their benefits or reimbursement packages can also influence use and will be based on the insurers' own reviews or other external reviews.
ENSURING THE QUALITY OF LABORATORIES PERFORMING GENETIC TESTS
Although laboratories performing chromosomal, biochemical, and/or DNA-based tests for genetic diseases must comply with general regulations under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), current requirements under CLIA are inadequate to ensure the overall quality of genetic testing because they are not specifically designed for any genetic tests except cytogenetic tests. Most laboratories performing genetic tests voluntarily participate in quality programs addressed specifically to genetic tests, but they are not required to do so. Consequently, providers and consumers have no assurance that every laboratory performs adequately.
Principles for Laboratories Adopting New Genetic Tests
No clinical laboratory should offer a genetic test whose clinical validity has not been established, unless it is collecting data on clinical validity under either an IRB-approved protocol or conditional premarket approval agreement with FDA (one of the options presented in chapter 2. The service laboratory should justify and document the basis of decisions to put new tests into service.
Regardless of where the test to be adopted was developed, clinical laboratory directors are responsible for ensuring the analytic validity of each genetic test their laboratory intends to offer before they make the test available for use in clinical practice (outside of an investigative protocol).Before routinely offering genetic tests that have been clinically validated, a laboratory must conduct a pilot phase in which it verifies that all steps in the testing process are operating appropriately.
If the pilot study reveals that the laboratory is not as competent as other laboratories in performing the test, or the test does not detect as many people with the genetic alteration as anticipated, the laboratory should not proceed to report patient-specific results without attempting to rectify the problems.Requirements Under CLIA
The stringency of CLIA requirements depends on the complexity level and specialty to which tests are assigned.
Complexity Ratings. CDC assigns a complexity level to a test according to predetermined criteria. Laboratories performing high complexity tests have more stringent personnel and quality-control requirements. Despite multiple uses, a test method gets only one rating. The Task Force recommends that tests that can be used for purposes of predicting future disease be given a rating of high complexity.
CLIA Specialties. Laboratories can perform tests only in specialties for which they are certified. Although there is a cytogenetics specialty, there is no genetics specialty. The Task Force welcomes the intention of CDC to create a genetics subcommittee of the Clinical Laboratory Improvement Advisory Committee (CLIAC), which advises on policies under CLIA. The Task Force urges this subcommittee to consider the creation of a specialty of genetics that would encompass all predictive genetic tests that satisfy criteria for stringent scrutiny. If a specialty of genetics is not feasible, the subcommittee should consider a specialty or subspecialty of molecular genetics for DNA/RNA-based tests. In the latter case, it must then address how to ensure the quality of laboratories performing nonDNA/RNA genetic tests. The subcommittee should also consider assigning tests that have widely different uses to more than one specialty.
Laboratory Personnel. Personnel requirements under CLIA, particularly at the level of laboratory director, depend on the specialty and complexity categories to which tests or analytes are assigned. Without a genetics specialty, genetic tests fall into other specialties for which requiring special training in genetics would be superfluous. The Task Force recommends that, for laboratories performing high complexity tests in the proposed specialty of molecular genetics, as well as in biochemical genetics and cytogenetics, personnel serving as directors or technical supervisors must have formal training in human and medical genetics, as documented by holding certification from an organization that assesses knowledge of human and medical genetics as part of its certification process, such as the American Board of Medical Genetics. Training programs for laboratory technicians/technologists need more human and medical genetics content than are currently available in the U.S.
Monitoring Laboratory Performance
Because laboratories provide services to providers and patients in many states it is clearly more desirable to have a rigorous Federal standard for certification or accreditation than fifty different State standards. Moreover, interstate genetic testing is unavoidable when only one or a few laboratories in the country provide tests. A national accreditation program for laboratories performing genetic tests, which includes proficiency testing and onsite inspection, is needed to promote standardization across the country. Such an accreditation program can occur more readily if a genetics specialty were established under CLIA. Until such time as a genetics specialty is established under CLIA, laboratories performing DNA/RNA-based tests for predictive purposes should choose to voluntarily participate in the College of American Pathologists' (CAP) molecular pathology program, including the CAP/American College of Medical Genetics (ACMG) molecular genetics proficiency testing program. Laboratories performing genetic tests on analytes not covered in the CAP/ACMG program, such as Tay-Sachs carrier screening and newborn screening, should participate in the available proficiency programs.
Proficiency Testing (PT). Under CLIA, every laboratory performing moderate or high complexity tests is required to enroll in PT programs recognized by HCFA. Any laboratory that fails a proficiency test must take corrective action.
So far, the Department of Health and Human Services has not approved proficiency testing programs for genetic tests because such tests do not measure regulated analytes for PT purposes. Nevertheless, under CLIA, laboratories must establish the accuracy and reliability of a test by methods of their own choosing. This can include participation in one of the voluntary PT programs. As these programs have not been approved by CLIA, no laboratory is obliged to use them and can establish accuracy and reliability by another method, although it must make the data available for onsite inspection under CLIA (see below).
Participation in well-established proficiency testing programs for genetic tests must be required under CLIA once a genetics specialty is established. When no relevant proficiency testing programs exist, laboratories must, whenever possible, participate in inter-laboratory comparison programs and help develop them if none exist in their particular area of testing. Proficiency testing programs should be broadly based since the number of genetic disorders is very large and the analytical approaches to testing are numerous.
Onsite Inspection
. All CLIA-certified laboratories are routinely inspected on a two-year survey cycle by (1) HCFA regional offices and State agencies, (2) private non-profit organizations to which HCFA has given "deemed" status in recognition of their ability to provide reasonable assurance that the laboratories they accredit meet the conditions required by Federal law, or (3) State-exempt licensure programs.CAP has deemed status to conduct inspections in several specialties, but since genetics is not a specialty under CLIA, the CAP program does not have deemed status in genetics. In the CAP genetics program, laboratories who voluntarily participate in the program are inspected.
Making Laboratory Performance Assessments Public
Publishing the names of laboratories performing satisfactorily would advise users that labs not appearing on the list have either not submitted to external review or have not performed adequately. HCFA annually publishes a list ("Laboratory Registry") that identifies all poor performance laboratories. As CAP is not deemed to accredit in areas of genetics, it does not make the results of its assessments of genetic test performance public. The Task Force recommends that CAP/ACMG periodically publish, and make available to the public, a list of laboratories performing genetic tests satisfactorily under its voluntary program. Other PT programs should also publish the names of laboratories performing satisfactorily if they do not already do so. Directories of laboratories providing genetic tests should also publish information on listed laboratories' satisfactory participation in PT and other quality control programs specific to genetic tests. Managed care organizations and other third-party payers should limit reimbursement for genetic tests to the laboratories on published lists of those satisfactorily performing genetic tests.
A Central Repository of Cell Lines and DNA
Making cell lines or DNA containing disease-related mutations available to many laboratories would be useful in the validation of new tests, calibration, standardization, and quality control. To accomplish this, appropriate specimens from patients, carriers, and controls should be available through a centralized repository in order to facilitate their availability to aid in analytical validation, improving quality, and other needs.
The Importance of the Pre- and Post-analytic Phases of Testing
Educational and promotional material made available by laboratories is often used by providers and consumers who are considering testing. The completeness and accuracy of this material is, therefore, extremely important. Obtaining informed consent helps ensure that the person voluntarily agrees to testing and has some understanding of the reasons for testing. The Task Force is of the opinion that laboratories should obtain documentation of informed consent when appropriate and should not perform an analysis if documentation is lacking.
Increasingly, genetic tests will be requested by providers without much or any training in genetics. Genetic test results must be written by the laboratory in a form that is understandable to the non-geneticist health care provider.
The Task Force recommends that CAP and ACMG seek advice and input from consumer groups, such as the Alliance of Genetic Support Groups, as well as from the National Society of Genetic Counselors (NSGC), on educational, psychological, and counseling issues in pre- and post-analytic components of genetic testing that are of direct concern to consumers. CDC should consider how the pre- and post-analytic phases of predictive genetic testing can be given greater weight in CLIA standards and regulations.
Direct Marketing of Genetic Tests to the Public
Many clinical laboratories advertise the availability of tests directly to the public. Great care must be taken that information on genetic tests presented directly to the public is accurate and includes risks and limitations, as well as benefits. Consumers should discuss testing options with a health care provider competent in genetics prior to having specimens collected for analysis. The Task Force discourages advertising or marketing of predictive genetic tests to the public.
International Harmonization
The Task Force recommends that efforts should be made to harmonize international laboratory standards to ensure the highest possible laboratory quality for genetic tests.
IMPROVING PROVIDERS' UNDERSTANDINGS OF GENETIC TESTING
The rate of increase of health care professionals trained and board-certified in medical genetics or genetic counseling has not kept pace with the rate of increase of genetic discovery and of potential demand for genetic tests. Other health care professionals will have to play a role or new models of testing will have to be devised if the demands are to be met.
A Role for Non-genetic Health Care Professionals
With adequate knowledge of test validity, disease and mutation frequencies in the ethnic groups to whom they provide care, primary care providers and other non-genetic specialists can and should be the ones to offer predictive genetic tests to at-risk individuals. The role of non-genetic providers in interpreting test results is complex. The interpretation of positive results will often depend on further elicitation of risks, including family history. The options available to reduce risks must also be known. Often the results will be of importance to other relatives. A test's sensitivity and predictive value may also vary by ethnic group. Providers must be aware of these and other considerations in interpreting test results and be capable of communicating risk information and its implications to those who are tested or their parents or guardians. Consultation with geneticists and/or genetic counselors may be appropriate.
Policies for Improving the Abilities of Non-genetic Health Care Professionals
Greater Public Knowledge of Genetics. A knowledge base on genetics and genetic testing should be developed for the general public. Without a sound knowledge base, informed decisions are impossible and claims of autonomy and informed consent suspect. People who are more knowledgeable will grasp more readily the issues raised by providers when they offer tests. This could diminish the time needed for education and counseling without reducing consideration of the implications of testing.
New models of providing education and counseling to patients and other consumers are needed.Undergraduate and Graduate Medical Education
. The Task Force encourages the development of genetics curricula in medical school and residency training to enable all physicians to recognize inherited risk factors in patients and families and appreciate issues in genetic testing and the use of genetic services. Those responsible for education and training have begun to recognize that most medical care is provided in ambulatory settings and that the delivery of care in those areas presents challenges for education. Genetic testing is a prime example. Moreover, teaching about genetic tests, including such issues as analytic and clinical validity, introduces students and residents to general problems of reliability and test sensitivity and specificity, which are important for a much wider range of clinical laboratory tests.Licensure and Certification.
The likelihood that genetics will be covered in curricula will improve if relevant genetics questions are included in general licensure and specialty board certification examinations, and if correctly answering a proportion of the genetics questions is needed to attain a passing score.Continuing Medical Education
. The full beneficial effects of improving medical school and residency curricula in genetics will not be felt for many years. Consequently, improving the ability of providers currently in practice to offer and interpret genetic tests correctly is of paramount importance. In addition to the basic curricula already considered, the Task Force recommends that each specialty involved with the care of patients with disorders with genetic components should design its own curriculum for continuing education in genetics.Administrators and other nonphysician personnel who triage patients and/or make coverage or reimbursement decisions, such as those in managed care organizations, should also have knowledge of the benefits and risks of genetic testing.
The Task Force endorses the recent establishment of a National Coalition for Health Professional Education in Genetics (NCHPEG) by the American Medical Association, the American Nurses Association, and the National Human Genome Research Institute
. In order to avoid duplication, the Coalition should serve as a registry and clearinghouse for, and disseminator of, information about various curricula and educational programs, grants, and training pilot programs in genetics education. It should encourage professional societies to track the effectiveness of their respective educational programs.A major problem in all educational endeavors is finding the "teachable moment," the time at which people, including health care providers, are receptive to new information and are most likely to retain it. These moments arise when providers are asked questions about genetic tests or when charts are flagged because the patient fulfills criteria for being offered a genetic test. To make information available at the teachable moments, a 1-800 hotline that providers can call to learn more about specific genetic tests should be encouraged by NCHPEG .
Demonstrating Provider Competence.
Hospitals and managed care organizations, on advice from the relevant medical specialty departments, should require evidence of competence before permitting providers to order predictive genetic tests defined as needing stringent scrutiny or to counsel about them. Periodic, systematic medical record review, with feedback to providers, should also be used to ensure appropriate use of genetic tests. In order to succeed, this policy requires, first, deciding which tests need evidence of competence, second, defining competence for those tests, and third, making educational modules readily available to enable providers to gain competence.Medical record audits assure managed care and other organizations that providers are satisfying standards of care. The feedback given to providers also serves as a valuable reenforcement to what has previously been learned. Audits of records for frequently-ordered medical tests should be considered.
Other Models
Nursing. Nurses have much to offer in helping people before, during, and after the genetic testing process. Because of their vast numbers and the wide range of health care activities they can perform, they can play an important role in providing care for those undergoing genetic testing. Nurses should be provided with additional education and training that can increase their effectiveness in providing education for people undergoing genetic testing.
Community and Public Health. Although population-wide screening can be integrated into personal health care, different models have been used. In many states, it is the responsibility of the hospital in which the baby is born to conduct newborn screening. As tests for more inherited conditions become available and the safety and effectiveness of treating them neonatally is established, newborn screening could expand markedly.
Community-centered screening presents another model. Tay-Sachs carrier screening was originally organized at the community level. Any effort to initiate community-based genetic screening must have the support and involvement of the community. Particularly when minority communities are involved, the program must be sensitive to issues of discrimination and provide sufficient resources for education and counseling.
Screening could be offered in health department clinics, mobile vans or other sites, but not all segments of the population are likely to utilize them. A greater chance of breaching confidentiality is possible at community and health department sites than in the privacy of the traditional provider-patient relationship. Traditionally, health departments have been most involved in clinical care when there were well-accepted interventions (such as immunizations or tuberculosis control) without which the health of the public would be jeopardized. It might be difficult for public health personnel to appreciate that someone who refuses genetic screening is not jeopardizing the health of the public. Before these new models are investigated, additional training of the personnel involved is necessary.
Schools of nursing, public health, and social work need to strengthen their training programs in genetics.
GENETIC TESTING FOR RARE INHERITED DISORDERS
Between 10 and 20 million Americans may suffer from one or more of the several thousand known rare diseases over their lifetimes. With the discovery of the role of inherited mutations in common diseases, such as breast and colon cancer and Alzheimer disease (albeit in a small proportion of affected people), the development and maintenance of tests for rare genetic diseases must continue to be encouraged. A comprehensive system to collect data on rare diseases must be established. Multiple sources will almost always be needed to validate tests for rare diseases.
CDC and the NIH Office of Rare Diseases (ORD) should work closely to develop the appropriate data-gathering and monitoring systems to assess the validity of genetic tests for rare diseases.Dissemination of Information About Rare Diseases
Unfortunately, the diagnosis of rare diseases is often delayed. One reason for the delay is inaccessibility of information. Physicians who encounter patients with symptoms and signs of rare genetic diseases should have access to accurate information that will enable them to include such diseases in their differential diagnosis, to know where to turn for assistance in clinical and laboratory diagnosis, and to locate laboratories that test for rare diseases.
Several private and public organizations, both professional and consumer-oriented, do provide information on rare diseases. The Task Force is concerned that there might be some unnecessary duplication of effort in compiling databases while, at the same time, some diseases or laboratories offering tests will not be included.
In order to avoid redundancy and to use the expertise of these organizations more efficiently, NIH should assign its Office of Rare Diseases (ORD) the task of coordinating these efforts and provide ORD with sufficient funds to fulfill the Task Force's recommendations on rare diseases. ORD should periodically report to the proposed Secretary's Advisory Committee on the status of these activities. With CDC playing a greater role in genetics, it should be closely involved in activities in this area.Ensuring Continuity and Quality of Tests for Rare Diseases
Because of the rarity of many diseases, only one or a few laboratories in the United States, or the world, accurately perform tests for some of them. To maintain and expand its database, ORD should identify laboratories worldwide that perform tests for rare genetic diseases, the methodology employed, and whether the tests they provide are in the investigational stage, or are being used for clinical diagnosis and decision making.
Some clinical diagnostic tests for rare diseases are performed in laboratories that are primarily engaged in research at no cost to the patient and with the primary purpose of furthering research. Such laboratories may cease performing these tests, on which clinical decisions are based, as they complete their investigations and move on to other areas of interest. The NIH Office of Rare Diseases should have the lead responsibility in ensuring the continued availability of safe and effective tests for rare diseases when it learns that a test will cease being offered. Funds to enable it to accomplish this task should be available.
Ensuring the Quality of Genetic Tests for Rare Diseases
In accordance with current law, the Task Force recommends that any laboratory performing any genetic test on which clinical diagnostic and/or management decisions are made should be certified under CLIA.
Research laboratories that are not currently providing genetic test results to providers or patients but that plan to do so in the future must register under CLIA. Once a laboratory registers, it does not have to wait for a survey before performing clinical tests.Research laboratories that provide physicians with results of genetic tests, which may be used for clinical decision making, must validate their tests and be subject to the same internal and external review as other clinical laboratories. Nevertheless
, the proposed genetics subcommittee of CLIAC should consider developing regulatory language under the proposed genetics specialty that is less stringent, but does not sacrifice quality for laboratories that only occasionally and in small volume perform tests whose results are made available to health care providers or patients.Directories of laboratories that perform tests for rare genetic diseases should indicate whether or not the laboratory is CLIA-certified and whether it has satisfied other quality assessment and proficiency assessments, such as those provided by CAP and ACMG. Directors of these laboratories are encouraged to participate in these programs or other programs of at least comparable quality that may be established.
Of great concern to the Task Force is whether certification under CLIA will ensure the quality of genetic tests, particularly those for rare genetic diseases. The creation of a subspecialty of genetics under CLIA will greatly improve the situation. Many tests for rare disorders are biochemical. The quality of performance of these tests would be ensured if they were included under a genetics specialty.
The principles and recommendations of the Task Force will help ensure that genetic testing will be provided safely and effectively and that tests for rare diseases will be more widely available but used appropriately. The Task Force concludes that with implementation of these recommendations, genetic testing will continue to flourish.
CHAPTER 1. INTRODUCTION
The remarkable advances in genetics in recent decades are the fruition of almost a century of basic research. Our ability to identify the underlying defects in single-gene (Mendelian) diseases, most of which are rare, has improved diagnosis in symptomatic individuals, and the prediction of risks of future disease in asymptomatic individuals. We
have learned how to prevent a few of these diseases by early intervention and how to treat a few others after symptoms appear. Gene therapy, in which a normal gene is introduced into cells of patients with defective genes, is being investigated in over 1,000 individuals, including some with Mendelian disorders such as cystic fibrosis and adenosine deaminase deficiency.2We now know that a small percentage of people with common disorders have inherited rare, single mutations that make them much more susceptible to developing the disease. Occasionally, single mutations that markedly increase susceptibility to disease reach frequencies as high as 1% in some population groups;3 usually the combined frequency of all such mutations is under 5% of all those who will develop the disease. More common genetic variants (polymorphisms) less markedly increase susceptibility.
Over the past half century, scientists have discovered the existence of DNA polymorphisms in which the most common form (allele) occurs in no more than 99% of the population. We are beginning to learn that some of these polymorphisms are associated with increased risks of common diseases, but usually not to the same degree as the rare variants. Conversely, some forms of polymorphisms convey resistance to disease. Before disease develops in people with either predisposing rare variants or polymorphisms, other genetic and environmental factors must be present.
Genetic discovery can benefit people in other ways than by discovering the inherited components. In the case of cancer, scientists have learned that acquired (somatic) mutations play a significant role.16 By comparing the molecular genetic profiles of cells from diseased organs and tissues to the comparable normal cells, scientists are beginning to learn which gene functions have been altered and how they might affect the development of chronic conditions like osteoporosis and arthritis.17 With this knowledge, interventions can be devised to avert or treat the triggering events or treat the disease effectively in its early stages.
Despite this remarkable progress much remains unknown. The unknowns have a strong impact on genetic testing, particularly when it is used predictively in healthy or apparently healthy people.
It has proven far more difficult to devise a means of preventing or treating most Mendelian genetic diseases than to diagnose or predict increased risk of them. A "therapeutic gap" exists. · No effective interventions are yet available to improve the outcome of most inherited diseases.
· Negative (normal) test results might not rule out future occurrence of disease. In the case of single-gene disorders, some tests do not detect all of the mutations capable of causing disease. In the case of common disorders, the disease often occurs even when tests for inherited susceptibility mutations or predisposing polymorphisms are negative.
· Positive test results might not mean the disease will inevitably develop. This is particularly a problem for the common disorders. For those who get the disease, the age at which it occurs and its severity and response to treatment cannot always be predicted. These problems arise in some Mendelian disorders, as well as in the common disorders. For instance, the severity of the lung disease, the most life-threatening aspect of cystic fibrosis, cannot be predicted by the mutations a person with CF possesses.22
It is primarily in the context of their unknown potential risks and benefits that the Task Force considers genetic testing.
Research and discovery in the first century of the next millennium will reduce the uncertainties, but the nature of human variation is such that it will never be possible to have genetic tests that are perfect predictors of disease. Even today, however, tests for the disorders for which these problems have not been solved can be of benefit.
· A negative test result in someone from a family in which affected relatives are known to have a disease-related mutation indicates a low risk of the disease. This can decrease anxiety and, for some diseases, reduce the frequency of periodic monitoring for early signs of the disease (e.g., mammography for breast cancer). A negative result can, depending on the disease, also enable a person to purchase health or life insurance at the standard rate.
· A positive test result enables a person to prepare for disease. Parents who learn from carrier screening that they are at risk of having an affected child can take steps to avoid the conception or birth of an affected child. People at risk of disease later in life can take steps to avoid passing the disease-causing allele on to their future children or can plan for the disease.
· Knowing that one is a carrier or has inherited a susceptibility to disease enables the person to inform relatives that they also might be at risk.
Nevertheless, problems will remain, especially as long as the means of preventing or treating genetic disease in those born with it are not fully at hand. The Task Force was created to make recommendations to ensure that genetic tests are safe and effective in view of the persistence of problems in the foreseeable future.
ORIGIN AND WORK OF THE TASK FORCE
In 1994, the National Institutes of Health (NIH)-Department of Energy (DOE) Working Group on Ethical, Legal, and Social Implications (ELSI) of Human Genome Research reviewed the report of the Institute of Medicine's Committee on Assessing Genetic Risks.23 Among the concerns raised in that report were the imperfect predictability of tests, the quality of laboratories providing clinical genetic tests, the lack of proven interventions for many disorders (see chapter 3), and the limited ability of many health care providers to explain genetic tests accurately and nondirectively to patients (see chapter 4). To consider these problems further, the Working Group convened the Task Force on Genetic Testing. It asked the Task Force to review genetic testing in the United States and, when necessary, make recommendations to ensure the development of safe and effective genetic tests. The Task Force has defined safety and effectiveness to encompass not only the validity and utility of genetic tests, but their delivery in laboratories of assured quality, and their appropriate use by health care providers and consumers.
How the public in general should be educated in genetics and genetic testing is beyond the purview of the Task Force, although it is critically important. So too, are policy recommendations--other than for improving genetic tests themselves--for reducing the harms that can result from some forms of genetic testing and can deter some people from being tested. Nevertheless, later in this chapter, the Task Force enunciates principles related to these harms.
The Working Group invited organizations with a stake in genetic testing to submit nominations from which it selected members of the Task Force. In addition, the Working Group invited five agencies in the Department of Health and Human Services (HHS) to send nonvoting liaison members to the Task Force. (Task Force members and their affiliations are listed at the front of this report.)
To determine the state of the art of genetic testing in the U.S., a survey of organizations likely to be engaged in genetic testing was undertaken for the Task Force
early in 1995. Following completion of the survey, in-depth interviews were conducted at 29 of the 463 organizations that indicated they were developing or providing genetic tests. Informational materials for providers and patients that were distributed by respondents who were performing genetic tests were collected and analyzed. Appendix 3 of the final report is a summary of the survey and interview findings, and appendix 4 is a summary of the analysis of the informational materials. The Task Force also commissioned papers on some of the more frequent genetic screening programs in the U.S. These appear in appendices 5 and 6. With the help of liaison representatives of relevant agencies and others, Task Force staff prepared analyses of various Federal statutes and regulations, most importantly those dealing with clinical laboratories and medical devices. Through notices in various genetics journals, an announcement on its World Wide Web page, and requests to consumer organizations, the Task Force asked professionals and consumers to report their experiences with various aspects of genetic testing. A small number of genetic counselors, physicians, and affected patients or their relatives responded. Some of these responses appear as sidebars throughout this report.In this report, all principles and recommendations of the Task Force appear in bold-faced type. Unfamiliar terminology can be found in the glossary.
The Task Force recognizes the tremendous potential of benefits from genetic testing. Its goal is to make recommendations that will assure the public that genetic tests will be safe and effective but will not stifle progress in this exciting field. It is particularly concerned about the continued availability of tests for rare inherited diseases.
The Task Force held seven meetings, all of which were open to the public. Halfway through its deliberations, the Task Force published Interim Principles,24 made them available on its World Wide Web site (http://ww2.med.jhu.edu/tfgtelsi), invited public comments, and held a public hearing on them. Taking these comments into consideration, the Task Force turned to developing recommendations to implement its principles. These were published in the Federal Register and also made available on the Web site.25 Once again, the public was given an opportunity to comment. A list of all organizations and persons commenting on the Interim Principles and Proposed Recommendations appears in appendix 1 of this report. The Task Force has taken these comments into consideration in preparing its final principles and recommendations.
DEFINITION OF GENETIC TESTS
The Task Force could not make recommendations on genetic tests without first defining them. After hearing considerable comment and much deliberation, the Task Force developed the following definition.
Genetic test--The analysis of human DNA, RNA, chromosomes, proteins, and certain metabolites in order to detect heritable disease-related genotypes, mutations, phenotypes, or karyotypes for clinical purposes. Such purposes include predicting risk of disease, identifying carriers, establishing prenatal and clinical diagnosis or prognosis. Prenatal, newborn, and carrier screening, as well as testing in high risk families, are included.
Tests for metabolites are covered only when they are undertaken with high probability that an excess or deficiency of the metabolite indicates the presence of heritable mutations in single genes. Tests conducted purely for research are excluded from the definition, as are tests for somatic (as opposed to heritable) mutations, and testing for forensic purposes.The Task Force is primarily concerned about predictive uses of genetic tests performed in healthy or apparently healthy people. Predictive test results do not necessarily mean that the disease will inevitably occur or remain absent; they replace the individual's prior risks based on population data or family history with risks based on genotype. The Task Force divides predictive tests into presymptomatic tests, which are performed to detect highly "penetrant" conditions, and predispositional tests, which are performed for incompletely penetrant conditions. The Task Force cannot limit its definition to predictive tests because some tests intended for diagnostic use can also be used predictively. The Task Force also decided that it cannot limit genetic tests only to those for which the analyte is DNA. Clinical laboratories will continue to use protein and enzyme and metabolite analyses for the purposes listed in the definition, including prediction.
Some, but not all, predictive genetic testing falls under the rubric "genetic screening." The Task Force follows the definition used in a National Research Council report: "Genetic screening may be defined as a search in a population for persons possessing certain genotypes that (1) are already associated with disease or predispose to disease, (2) may lead to disease in their descendants, or (3) produce other variations not known to be associated with disease." 26 (p. 9) Under this definition, testing an asymptomatic person in a family with several relatives affected with disease does not constitute screening but predictive genetic testing.
The Task Force rejected the suggestion from the College of American Pathologists (CAP) that, "The definition of genetic tests should focus on germ line mutations that require genetic counseling with respect to the development of diseases." Neither the Task Force nor any other body has stated which tests require genetic counseling. The Task Force did acknowledge the concerns of CAP and The American Society of Clinical Pathologists that too many tests in standard use would be covered by limiting its definition to tests for metabolites only when they are "undertaken with high probability that an excess or deficiency of the metabolite indicates the presence of heritable mutations in single genes". Under the definition, cholesterol screening in the general population would not be covered, but cholesterol testing in a family with a documented low density lipoprotein receptor defect would be covered. Newborn screening tests for metabolites whose excess or deficiency require followup to rule out a heritable disorder would be covered.
It is not the intention of the Task Force that all of its recommendations be applied to all tests that meet its definition. A system is needed to classify genetic tests according to the scrutiny they need. Later in this chapter, the Task Force suggests how such a system can be developed.
REVIEW OF GENETIC TESTING
Over 500 commercial, university, and health department laboratories provide tests for inherited and chromosomal disorders, and genetic predispositions in the United States. Virtually every newborn is screened for phenylketonuria and congenital hypothyroidism and many are screened for sickle cell disorders.27 Screening for carriers of Tay-Sachs and sickle cell is performed among populations at risk. Based on the recommendations of a recent consensus panel,28 cystic fibrosis carrier screening might increase. Approximately 2.5 million pregnant women are screened each year to see if their fetuses are at high risk of neural tube defects or Down syndrome.29 Of 467 organizations who responded fully to the survey conducted for the Task Force, 56.7% indicated that they were testing for at least one of 44 inherited conditions that were listed in the questionnaire (see appendix 3). A few commercial and university laboratories were offering tests for inherited susceptibility mutations to breast and colon cancer. Of 197 health maintenance organizations who responded to a recent survey, 45% said they were covering predictive tests for breast cancer and 42% were covering for colon cancer for some of their subscribers.30
For the most part, genetic testing in the United States has developed successfully, providing options for avoiding, preventing, and treating inherited disorders. However, there are some problems, which are spelled out in greater detail later in this report and in the appendices.
·
Sometimes, genetic tests are introduced before they have been demonstrated to be safe, effective, and useful (see chapter 2 and appendices 5 and 6).·
There is no assurance that every laboratory performing genetic tests for clinical purposes meets high standards (see chapter 3).·
Often, the informational materials distributed by academic and commercial genetic testing laboratories do not provide sufficient information to fill in the gaps in providers' and patients' understanding of genetic tests (see appendix 4).
THE NEED FOR RECOMMENDATIONS
In the past few years, scientific and professional societies, as well as consumer groups, have felt impelled to publicly express concern when predictive tests were introduced with insufficient evidence of safety and effectiveness. These included prenatal screening with alpha-fetoprotein and other markers,31,32 carrier screening for cystic fibrosis,33,34 testing for susceptibility to cancer35,36 and breast cancer in particular,37,38 and Alzheimer disease.39,40 These statements often expressed a reaction to the imminence or appearance of a test and undoubtedly reduced inappropriate use of tests. The publication of each statement depended on mobilizing individuals with interest and expertise and then getting ratification by the sponsoring organization, tasks not easily accomplished in a short period without extraordinary effort.
This becomes an impossible task as the number of tests expands but the problems persist.Although professional societies must play a major role in solving problems of genetic testing, they are only one of several stakeholders, some of whose interests conflict with others'. The Task Force believes that all stakeholders must be involved. As this report demonstrates, they often will succeed in resolving disagreements and reaching consensus.
Except for neonatal and prenatal screening and diagnosis, the volume of testing has not been great and much of the testing has been performed in genetic centers or in consultation with highly-trained geneticists and genetic counselors.
In the next few years, the use of genetic testing is likely to expand rapidly while the number of genetic specialists remains essentially unchanged. A greater burden for making genetic testing decisions will fall on providers who have little formal training or experience in genetics and are less equipped to deal with the complex and special problems raised by some predictive genetic tests. Consulted primarily by people who are sick, and who expect doctors to tell them what to do to get better, many physicians adopt a directive stance when asked how they would deal with genetic tests and results that have reproductive implications.Until the 1980s most genetic and cytogenetic testing was performed in the laboratories of non-profit organizations, most of them in academic medical centers. These labs were often directed by the same professionals who cared for patients. In the last decade, genetic testing has been commercialized. As a result, providers who were close to patients and families at risk of illness might not have as much influence on testing policy as they once did.
Although formal comparisons have not been made, there is little evidence that the problems encountered in the development and delivery of genetic testing technologies have been more frequent or severe than for other medical technologies. Some problems encountered in other specialties have not been trivial. Amendments to the Food, Drug and Cosmetic Act, and to the Clinical Laboratory Improvement Act were passed by Congress because of problems in the clinical use of some new medical technologies.41-45 In 1996, recognizing the challenge posed by genetic tests, two Congressional committees held hearings related to the validity and quality of genetic tests.46,47
The ELSI component of the Human Genome Project was founded on the concept that the new technologies of gene identification will engender problems that can be minimized if anticipated and dealt with promptly. The recommendations of the Task Force are very much in this vein. In this report, the Task Force does not recommend policies for specific tests but suggests a framework for ensuring that new tests meet criteria for safety and effectiveness before they are unconditionally released, thereby reducing the likelihood of premature clinical use.
The focus of the Task Force on potential problems in no way is intended to detract from the benefits of genetic testing. Its overriding goal is to recommend policies that will reduce the likelihood of damaging effects so testing's benefits can be fully realized undiluted by harm.
SCOPE OF THE REPORT
The Task Force has tried to stay within the limits of its charge and to use past and current genetic testing as its guide. In the remainder of this chapter we consider the need for a central advisory body on genetic testing, and enunciate overarching principles on problems that are not integral to genetic testing per se but impinge on, or that may arise as a consequence of, genetic testing. The next chapter considers criteria for the development of new genetic tests. It presents policies to ensure that sufficient evidence of the safety and effectiveness of new genetic tests is collected and is reviewed before tests are unconditionally made available for clinical use. In chapter 3, we consider how the quality of the laboratories that provide genetic testing to health care providers in clinical practice can be ensured. Because new tests are often developed in clinical laboratories, the chapter begins with a consideration of laboratories' responsibilities in developing new tests. In chapter 4, the expanding role of non-genetic health care providers in genetic testing is considered, followed by discussion of some of the obstacles to their providing testing appropriately. The chapter describes policies to ensure that providers who use genetic testing have an adequate understanding of the indications for genetic tests and their limitations. Chapter 5 raises several concerns about rare genetic diseases, which constitute the largest number of genetic diseases. Collectively rare diseases represent the most frequent indication for genetic testing. Policies for ensuring that providers include rare diseases when they consider the causes of some of their patients' problems and that they know how and where to obtain information about rare diseases, including where to obtain diagnostic and predictive clinical laboratory tests are considered. The chapter concludes with recommendations for ensuring the continuity and quality of clinical laboratory tests for rare diseases.
This report does not contain a separate chapter on genetic testing under public health auspices. The Task Force spent considerable time discussing this issue and concluded that its recommendations for genetic tests in clinical practice also apply to tests included in health department screening programs. Some members of the Task Force and several who submitted comments questioned the need for informed consent in public health programs that are undertaken only when the benefits to the individual markedly outweigh the risks. Task Force principles on this issue are presented later in this chapter. A public health role is discussed briefly in chapter 4.
NEED FOR AN ADVISORY COMMITTEE ON GENETIC TESTING
Policies related to genetic testing involve several different Federal agencies, as well as the private sector. Such policies can best be formulated and implemented by having input from many different sources in order to achieve the single goal: the availability of safe and effective genetic tests.
The Task Force calls on the Secretary of Health and Human Services to establish an advisory committee on genetic testing in the Office of the Secretary. Members of the committee should represent the stakeholders in genetic testing, including professional societies (general medicine, genetics, pathology, genetic counseling), the biotechnology industry, consumers, and insurers, as well as other interested parties. The various HHS agencies with activities related to the development and delivery of genetic tests should send nonvoting representatives to the advisory committee, which can also coordinate the relevant activities of these agencies and private organizations. The Task Force leaves it to the Secretary to determine the relationship of this advisory committee to others that may be created in the broader area of genetics and public policy, of which genetic testing is only one part.
The committee would advise the Secretary on implementation of recommendations made by the Task Force in this report to ensure that (a) the introduction of new genetic tests into clinical use is based on evidence of their analytical and clinical validity, and utility to those tested; (b) all stages of the genetic testing process in clinical laboratories meet quality standards; (c) health providers who offer and order genetic tests have sufficient competence in genetics and genetic testing to protect the well-being of their patients; and (d) there be continued and expanded availability of tests for rare genetic diseases.
The Task Force recognizes the widely inclusive nature of genetic tests. It is therefore essential that the advisory committee recommend policies for the Secretary's consideration by which agencies and organizations implementing recommendations can determine those genetic tests that need stringent scrutiny. Stringent scrutiny is indicated when a test has the ability to predict future inherited disease in healthy or apparently healthy people, is likely to be used for that purpose, and when no confirmatory test is available. The advisory committee or its designate should define additional indications.
In order to carry out its functions, the advisory committee should have its own staff and budget.
The Task Force further recommends that the Secretary review the accomplishments of the advisory committee on genetic testing after 2 full years of operation and determine whether it should continue to operate.
NOTE: Hereafter, the advisory committee on genetic testing is referred to as the proposed Secretary's Advisory Committee.
OVERARCHING PRINCIPLES
In making recommendations on safety and effectiveness, the Task Force concentrated on test validity and utility, laboratory quality, and provider competence. It recognizes, however, that other issues impinge on testing, and problems can arise from testing. Regarding these issues, the Task Force endorses the following principles.
Informed Consent
The Task Force strongly advocates written informed consent, especially for certain uses of genetic tests, including clinical validation studies and predictive testing. The failure of the Task Force to comment on informed consent for other uses does not imply that it should not be obtained.
Test Development. Informed consent for any validation study must be obtained whenever the specimen can be linked to the subject from which it came. As long as identifiers are retained in either coded or uncoded form, the possibility exists to contact subjects even if the intent of the original protocol was not to do so. As part of the disclosure for consent, individuals must be informed of possible future uses of the specimen, whether identifiers will be retained and, if so, whether the individual will be recontacted.
Testing in Clinical Practice. (1) It is unacceptable to coerce or intimidate individuals or families regarding their decision about predictive genetic testing. Respect for personal autonomy is paramount. People being offered testing must understand that testing is voluntary. Their informed consent should be obtained. Whatever decision they make, their care should not be jeopardized. Information on risks and benefits must be presented fully and objectively. A non-directive approach is of the utmost importance when reproductive decisions are a consequence of testing or when the safety and effectiveness of interventions following a positive test result have not been established. Obtaining written informed consent helps to ensure that the person voluntarily agrees to testing.
(2) Prior to the initiation of predictive testing in clinical practice, health care providers must describe the features of the genetic test, including potential consequences, to potential test recipients. Individuals considering genetic testing must be told the purposes of the test, the chance it will give a correct prediction, the implications of test results, the options, and the benefits and risks of the process. The responsibility for providing information to the individual lies with the referring provider, not with the laboratory performing the test.
Newborn Screening. (1) If informed consent is waived for a newborn screening test, the analytical and clinical validity and clinical utility of the test must be established, and parents must be provided with sufficient information to understand the reasons for screening. By clinical utility, the Task Force means that interventions to improve the outcome of the infant identified by screening have been proven to be safe and effective. Using newborn screening to identify couples who are at risk of having a future child with sickle cell anemia or other disorder because their screened infant is found to be a carrier (heterozygote) is not of primary benefit to the infant screened. Using newborn screening to identify parents at risk should only be done after this intention is communicated to parents (prior to screening) and their written consent is obtained. The Task Force recognizes that newborn screening programs have succeeded in significantly reducing the burden of a number of inherited disorders by timely diagnosis and institution of preventive therapies. Sometimes, however, newborn screening is undertaken before tests are validated and interventions are established to prevent or reduce clinical problems (see appendix 5). A recent consensus development conference on cystic fibrosis concluded that the evidence to warrant routine screening of newborns for cystic fibrosis was insufficient.28
(2) For those disorders for which newborn screening is available but the tests have not been validated or shown to have clinical utility, written parental consent is required prior to testing. The Task Force also recognizes that specimens collected for newborn screening become an important resource for developing new tests. When the infant's name or other identifying information is retained on these specimens, the Task Force believes that parental informed consent is needed.
Prenatal and Carrier Testing
Respect for an individual's/couples' beliefs and values concerning tests undertaken for assisting reproductive decisions is of paramount importance and can best be maintained by a nondirective stance. One way of ensuring that a non-directive stance is taken and that parents' decisions are autonomous, is through requiring informed consent.
Testing of Children
Genetic testing of children for adult onset diseases should not be undertaken unless direct medical benefit will accrue to the child and this benefit would be lost by waiting until the child has reached adulthood. The Task Force agrees with the American Society of Human Genetics and the American College of Medical Genetics that "Timely medical benefit to the child should be the primary justification for genetic testing in children and adolescents."48 Although sympathetic to the considerable difficulties inherent in living with uncertainty about the health status of the child, the Task Force does not feel that these warrant foreclosing the child's right to make an independent decision in regard to testing in adulthood. We are aware, however, that there are situations (e.g., testing for inherited mutations in the ademomatous polyposis coli gene) in which the benefit of avoiding medical surveillance (if the test result is negative) is sufficient to warrant testing even though no treatment will usually be undertaken until a later age (if the test result is positive). In addition, the Task Force realizes that legal adulthood is a somewhat arbitrary concept. For example, in families with a considerable burden of disease and in which several adults are undergoing genetic testing, older teenagers might request testing for themselves in order to reduce uncertainty and anxiety. It is unfortunate that almost no research evidence currently exists on the risks and benefits of genetic testing to teenagers and younger children. We believe that such psychosocial research must be pursued as vigorously as research on issues of analytic validity or utility of tests. However, unless and until such time as contradictory research findings emerge, testing of minors for presumed psychological benefits should be avoided.
Confidentiality
Protecting the confidentiality of information is essential for all uses of genetic tests.
(1) Results should be released only to those individuals for whom the test recipient has given consent for information release. Means of transmitting information should be chosen to minimize the likelihood that results will become available to unauthorized persons or organizations. Under no circumstances should results with identifiers be provided to any outside parties, including employers, insurers, or government agencies, without the test recipient's written consent. Consent given for minors should expire when the minor reaches adulthood.
Unless potential test recipients can be assured that the results will not be given to individuals or organizations they have not specifically named, some will refuse testing for fear of losing insurance, employment, or for other reasons. Aggregate results, stripped of identifiers, can be reported to government agencies for statistical and planning purposes.
(2) Health care providers have an obligation to the person being tested not to inform other family members without the permission of the person tested, except in extreme circumstances.
The Task Force agrees with recommendations of The President's Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research49 and the Institute of Medicine23 that
disclosure by providers to other family members is appropriate only when the person tested refuses to communicate information despite reasonable attempts to persuade him or her to do so, and when failure to give that information has a high probability of resulting in imminent, serious, and irreversible harm to the relative, and when communication of the information will enable the relative to avert the harm. When test results have serious implications for relatives, it is incumbent upon providers to explain to people who are tested the reasons why they should communicate the information to their relatives and to counsel them on how they should convey the information so the communication itself does not result in undue harm. Great care must be taken to avoid inadvertent release of information.Recently, a subcommittee of the American Society of Human Genetics50 endorsed these same principles for disclosure to relatives, but suggested that "the health care professional should be obliged to inform the patient of the implications of his/her genetic test results and potential risks to family members. Prior to genetic testing and again upon refusal to communicate results, this duty to inform the patient of familial implications is paramount. (emphasis added)." The Task Force is of the opinion that, as part of this duty, providers must make clear that they will not communicate results to relatives, except in extreme circumstances, which the provider should define. If left with the impression that the provider will inform relatives when the person considering testing does not want them informed, some people will decline testing. This would have the effect not only of denying information to the relative but to the person offered testing as well. Providers should be explicit in describing the extreme situations in which they would inform other relatives.
Harm can also result when relatives communicate genetic information. Strategies to assist individuals in communicating information to relatives should be developed.
Discrimination
No individual should be subjected to unfair discrimination by a third party on the basis of having had a genetic test or receiving an abnormal genetic test result. Third parties include insurers, employers, and educational and other institutions that routinely inquire about the health of applicants for services or positions. Discrimination can take the form of denial or of additional charges for various types of insurance, employment jeopardy in hiring and firing, or requirements to undergo unwanted genetic testing. Protection from unfair discrimination has been the subject of legislation at both the State and Federal levels.51 The problem has not been completely solved.52,53
Consumer Involvement in Policy Making
Although other stakeholders are concerned about protecting consumers, they cannot always provide the perspective brought by consumers themselves, the end users of genetic testing. Clearly, there are technical issues that cannot be decided primarily by consumers, but consumers must be involved in decision making on matters of policy in test development and in clinical use that directly affects their well-being. Consumers should be involved in policy (but not necessarily in technical) decisions regarding the adoption, introduction, and use of new, predictive genetic tests.
Issues Not Covered
There are aspects of genetic testing with which we have not dealt. Several respondents asked the Task Force to comment on genetic testing for non-medical conditions, such as homosexuality or other behavioral traits, or for gene enhancement. Although the Task Force has drawn upon examples of past and current testing, it has not made pronouncements about specific types of testing. As already stated, its intent is to develop generic policies that cover predictive testing for a wide range of medical conditions.
The Task Force recognizes that patenting and licensing can have a profound effect on the costs of medical tests. The payment of license fees is likely to be passed on to third-party payers or to consumers if they do not have or wish to use their health insurance. This issue has been highlighted recently by lawsuits by a patent holder to force laboratories performing prenatal screening for Down syndrome to pay royalties.54 The issue of patenting and licensing needs further exploration but is beyond the scope of the Task Force.
The Task Force has not dwelled in depth on the use of stored tissues for genetic research, including the development of genetic tests. Recommendations on this issue have been made by others55-58 and are still being actively discussed and modified.
Undoubtedly, others would have liked us to comment on additional issues. We reiterate that our main concern is the safety and effectiveness of genetic tests in both the developmental phase and the clinical-use phase. We turn now to these major topics.
REFERENCES
1. Scriver CR, Beaudet AL, Sly WS, Valle D, editors: The Metabolic and Molecular Bases of Inherited Disease. Seventh Edition. New York, McGraw-Hill, Inc. 1995.
2. Friedmann T: Overcoming the obstacles to gene therapy. Scientific American 1997;276:96-101.
3. Struewing JP, Hartge P, Wacholder S, et al: The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Askhenazi Jews. New England Journal of Medicine 1997;336:1401-1408.
4. Szabo CI, King M: Invited editorial: Population genetics of BRCA1 and BRCA2. American Journal of Human Genetics 1997;60:1013-1020.
5. Kinzler KW, Vogelstein B: Lessons from hereditary colorectal cancer. Cell 1996;87:159-170.
6. Morrison-Bogorad M, Phelps C, Buckholtz N: Alzheimer disease research comes of age. The pace accelerates. JAMA 1997;277:837-840.
7. Seshadri S, Drachman DA, Lippa CF: Apolipoprotein E e4 allele and the lifetime risk of Alzheimer's disease. What physicians know, and what they should know. Archives of Neurology 1995;52:1074-1079.
8. Tisch R, McDevitt H: Insulin-dependent diabetes mellitus. Cell 1996;85:291-297.
9. Vyse TJ, Todd JA: Genetic analysis of autoimmune diseases. Cell 1996;85:311-318.
10. Ridker PM, Miletich JP, Hennekens CH, Buring JE: Ethnic distribution of Factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA 1997;277:1305-1307.
11. Frosst P, Blom HJ, Milos R, et al: A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nature Genetics 1995;10:111-113.
12. Reynolds MV, Bristow MR, Bush EW, et al: Angiotensin-converting enzyme DD genotype in patients with ischaemic or idiopathic dilated cardiomyopathy. Lancet 1993;342:1073-1075.
13. Nebert DW: Polymorphisms in drug-metabolizing enzymes: What is their clinical relevance and why do they exist? American Journal of Human Genetics 1997;60:265-271.
14. Smith MW, Dean M, Carrington M, et al: Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Science 1997;277:959-968.
15. Bell J: The new genetics of clinical practice. BMJ 1997;(In Press).
16. Vogelstein B, Kinzler KW: The multistep nature of cancer. Trends in Genetics 1993;9:138-141.
17. Haseltine WA: Discovering genes for new medicine. Scientific American 1997;276:92-97.
18. Treacy E, Childs B, Scriver CR: Response to treatment in hereditary metabolic disease: 1993 survey and 10-year comparison. American Journal of Human Genetics 1995;56:359-367.
19. Burke W, Petersen G, Lynch P, et al: Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. JAMA 1997;277:915-919.
20. Schrag D, Kuntz KM, Garber JE, Weeks JC: Decision analysis -- effects of prophylactic mastectomy and oophorectomy on life expectancy among women with BRCA1 or BRCA2 mutations. New England Journal of Medicine 1997;336:1465-1471.
21. Burke W, Daly M, Garber J, et al: Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. JAMA 1997;277:997-1003.
22. Cystic Fibrosis Genotype-Phenotype Consortium: Correlation between genotype and phenotype in patients with cystic fibrosis. New England Journal of Medicine 1993;329:1308-1313.
23. Andrews L, Fullarton JE, Holtzman NA, Motulsky AG, eds. Assessing genetic risks: Implications for health and social policy. Washington DC, National Academy Press; 1994.
24. Task Force on Genetic Testing: Interim principles. Available at www.med.jhu.edu/tfgtelsi 1996.
25. National Institutes of Health: Proposed recommendations of the Task Force on Genetic Testing; Notice of meeting and request for comment. Federal Register 1997;62:4539-4547.
26. Committee for the Study of Inborn Errors of Metabolism: Genetic screening: Programs, principles, and research. Washington DC, National Academy of Sciences; 1975.
27. Hiller EH, Landenburger G, Natowicz MR: Public participation in medical policy making and the status of consumer autonomy: The example of newborn screening programs in the United States. American Journal of Public Health 1997;87(8):1280-1288.
28. Howell RR, Borecki I, Davidson ME, et al: National Institutes of Health Consensus Development Conference Statement: Genetic testing for cystic fibrosis. 1997;in press.
29. Palomaki GE, Knight GJ, McCarthy JE, Haddow JE, Donhowe JM: Maternal serum screening for Down syndrome in the United States: A 1995 survey. American Journal of Obstetrics and Gynecology 1997;176:1046-1051.
30. Myers MF, Doksum T, Holtzman NA: Coverage and provision of genetic services: Surveys of health maintenance organizations (HMOs) and academic genetic units (AGUs). American Journal of Human Genetics 1997;in press. (Abstract)
31. Council on Scientific Affairs: Maternal serum a-fetoprotein monitoring. JAMA 1982;247:1478-1481.
32. American Society of Human Genetics: Maternal serum alpha-fetoprotein screening programs and quality control for laboratories performing maternal serum and amniotic fluid alpha-fetoprotein assays. American Journal of Human Genetics 1987;40:75-82.
33. American Society of Human Genetics: The American Society of Human Genetics Statement on cystic fibrosis screening. American Journal of Human Genetics 1990;46:393.
34. National Institutes of Health: Statement from the National Institutes of Health Workshop on population screening for the cystic fibrosis gene. New England Journal of Medicine 1990;323:70-71.
35. National Advisory Council for Human Genome Research: Statement on use of DNA testing for presymptomatic identification of cancer risk. JAMA 1994;271:785.
36. American Society of Clinical Oncology: Statement of the American Society of Clinical Oncology: Genetic testing for cancer susceptibility, Adopted on February 20, 1996. Journal of Clinical Oncology 1996;14:1730-1736.
37. American Society of Human Genetics Ad Hoc Committee: Statement of The American Society of Human Genetics on genetic testing for breast and ovarian cancer predisposition. American Journal of Human Genetics 1994;55(5):i-iv.
38. National Breast Cancer Coalition. Presymptomatic genetic testing for heritable breast cancer risk. Washington DC, 1995.
39. American College of Medical Genetics: Statement on use of apolipoprotein E testing for Alzheimer disease. JAMA 1995;274:1627-1629.
40. National Institute on Aging: Apolipoprotein E genotyping in Alzheimer's disease. Lancet 1996;347:1091-1095.
41. Higgs R: Hazardous to our health? FDA regulation of health care products. Oakland, Independent Institute; 1995.
42. Merrill RA: Regulation of drugs and devices: An evolution. Health Affairs 1994;Summer:46-69.
43. Bogdanich W: False negative. Medical labs, trusted as largely error-free, are far from infallible. Wall Street Journal Feb. 2, 1987:1.
44. Bogdanich W: Risk factor. Inaccuracy in testing cholesterol hampers war on heart disease. Wall Street Journal Feb. 3, 1987:1.
45. Nash P: Discussion Session I. Clinical Chemistry 1992;38:1220-1222.
46. Subcommittee on Technology, Committee on Science, U.S. House of Representatives Hearing on Technological advances in genetics testing: Implications for the future. 1996.
47. U.S.Senate Committee on Labor and Human Resources. Hearing on Advances in Genetics Research and Technologies: Challenges for Public Policy. 1996.
48. American Society of Human Genetics, American College of Medical Genetics: Points to consider: Ethical, legal, and psychosocial implications of genetic testing in children and adolescents. American Journal of Human Genetics 1995;57:1233-1241.
49. President's Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research: Screening and Counseling for Genetic Conditions. Washington DC, U.S. Government Printing Office; 1983.
50. American Society of Human Genetics Social Issues Sub-Committee on Familial Disclosure: Professional disclosure of familial genetic information. American Journal of Human Genetics 1997;in press.
51. Rothenberg KH: Genetic information and health insurance: State legislative approaches. Journal of Law, Medicine & Ethics 1995;23:3112-319.
52. Hudson KL, Rothenburg KH, Andrews LB, Kahn MJE, Collins FS: Genetic discrimination and health insurance: An urgent need for reform. Science 1995;270:391-393.
53. Rothenberg KH, Fuller B, Rothstein M, et al: Genetic information and the workplace: Legislative approaches and policy challenges. Science 1997;275:1755-1757.
54. Eichenwald K: Push for royalties threatens use of Down Syndrome test. New York Times May 23, 1997;A1.
55. Clayton EW, Steinberg KK, Khoury MJ, et al: Informed consent for genetic research on stored tissue samples. JAMA 1995;274:1786-1792.
56. American College of Medical Genetics: ACMG Statement. Statement on storage and use of genetic materials. American Journal of Human Genetics 1995;57:1499-1500.
57. American Society of Human Genetics: ASHG report. Statement on informed consent for genetic research. American Journal of Human Genetics 1996;59:471-474.
58. Academy for Clinical Laboratory Physicians and Scientists, et al. Uses of human tissue. August 28, 1996. 1996;draft.
CHAPTER 2. ENSURING THE SAFETY AND EFFECTIVENESS OF NEW GENETIC TESTS
Some predictive genetic tests become available without adequate assessment of their benefits and risks. When this happens, providers and consumers cannot make a fully-informed decision about whether or not to use them. Although extensive use has eventually proved most tests to be of benefit, a few have not proved helpful and were discarded or modified. In the meantime, people were wrongly classified as at-risk and subjected to treatments that, in their case, proved unnecessary or sometimes harmful. Others, who could have benefited from treatment were classified as "normal" and not treated. Harmful effects can be avoided or at least reduced if systematic, well-designed studies to assess a test's safety and effectiveness are undertaken before tests become routinely available and after they are significantly modified. In this chapter, we present criteria for assessing genetic tests prior to routine use, policies for ensuring that the necessary data are collected and, finally, recommendations for review of the data before tests are routinely used.
CRITERIA FOR DEVELOPING GENETIC TESTS
The Task Force strongly holds that the clinical use of a genetic test must be based on evidence that the gene being examined is associated with the disease in question, that the test itself has analytical and clinical validity, and that the test results will be useful to the people being tested. In this section, we first describe these criteria and then consider how adherence to them can be ensured.
Establishing Associations Between a Disease, Genes, and Inherited Mutations
In developing genetic tests, scientists must first be confident that the DNA segments under investigation play a role in the disease in question. These segments might be apparently functionless markers that appear to be spatially linked on a chromosome to a disease-related gene. Linkage is demonstrated when, within families, one form of the marker is found in those with the disease more often than in blood relatives in whom the disease is absent. Because such associations might be due to chance,
as was the case for the linkage claimed between bipolar affective disorder and markers on chromosome 11, and between schizophrenia and markers on chromosome 5,1,2 stringent statistical standards must be satisfied before accepting linkage,3 and the findings must be confirmed in additional families with the disease. The method has proved successful in locating disease-related genes for Huntington disease, cystic fibrosis, breast cancer, and other disorders.Further research leads scientists from the linked, functionless marker to a nearby gene suspected of being causally related to the diseases in question. The proof depends on finding mutations in the gene that are only present (in gene dosage sufficient to cause disease) in family members with disease.
a Further proof that a gene is causally related to disease comes from demonstrating that the protein encoded by the gene is absent, not synthesized in adequate amounts, or manifests a structural or functional aberration that plausibly accounts for symptoms and signs of the disease.Another approach to identifying a disease-related gene does not depend on linkage but on suspecting that a gene that has been previously identified ("candidate" gene) plays a role in a specific disease. Here too, mutations (in gene dosage sufficient to cause disease) must be found only in those with the disease.
The DNA segments associated with a disease might be functional, common, polymorphic gene variants. Recently, attention has been given to the association between the apolipoprotein E polymorphism and Alzheimer disease (AD).7 A higher proportion of people with apoE4 will develop AD than those with other forms of the polymorphism. Some people with AD, however, will not inherit apoE4 and others with apoE4 will never develop AD;8 the polymorphism is neither a necessary nor sufficient cause for the disease. It is not clear whether polymorphic variants themselves predispose to the disease, whether the association is spurious (unlikely in the case of apoE4 and AD), or whether a marker linked to both the polymorphic gene and the disease-related gene is responsible.
b The following criterion must be satisfied before either linked markers or putative disease-related mutations are used as the basis of a genetic test. The genotypes to be detected by a genetic test must be shown by scientifically valid methods to be associated with the occurrence of a disease. The observations must be independently replicated and subject to peer review.Analytical Validity
For DNA-based tests, analytical validity requires establishing the probability that a test will be positive when a particular sequence (analyte) is present (analytical sensitivity) and the probability that the test will be negative when the sequence is absent (analytical specificity).
c In contrast to DNA-based tests, enzyme and metabolite assays measure continuous variables (enzyme activity or metabolite concentration). One key measure of their analytical validity is accuracy, or the probability that the measured value will be within a predefined range of the true activity or concentration. Another measure of analytical validity is reliability, or the probability of repeatedly getting the same result.Analytical validation of a new genetic test includes comparing it to the most definitive or "gold standard" method. The first genetic test to be used clinically might, however, be the gold standard; for example, a test that employs sequencing to detect disease-related mutations. In either case, validation includes performing replicate determinations to ensure that a single observation is not spurious, and "blind" testing of coded positive samples (from patients with the disease in whom the alteration is known to be present) and negative samples (from controls). Organizations engaged in new test development should have access to a sufficient number of patient samples to have statistical confidence in the validation. In validating a new test analytically, the laboratory techniques should be as similar as possible to those used when the test will be performed clinically once it is validated.
Analytical sensitivity and specificity of a genetic test must be determined before it is made available in clinical practice.
Clinical Validity
Clinical validation involves establishing several measures of clinical performance including (1) the probability that the test will be positive in people with the disease (clinical sensitivity), (2) the probability that the test will be negative in people without the disease (clinical specificity), and (3) the probability that people with positive test results will get the disease (positive predictive value (PPV)) and that people with negative results will not get the disease (negative predictive value). Predictive value depends on the prevalence of the disease in the group or population being studied, as well as on the clinical sensitivity and specificity of the test.
Two intrinsic features of genetic diseases, heterogeneity and penetrance, affect clinical validity.
Heterogeneity. The same genetic disease might result from the presence (in the necessary gene dosage) of any of several different variants (alleles) of the same gene (allelic diversity) or of different genes (locus heterogeneity). With current technology, all disease-related alleles cannot always be identified, particularly when there are many of them, which is often the case. This failure to detect all disease-related mutations reduces a test's clinical sensitivity.
Penetrance. The probability that disease will appear when a disease-related genotype is present is the penetrance of the genotype. When penetrance is incomplete, PPV is reduced. Penetrance is incomplete when other genetic or environmental factors must be present. In high-risk breast cancer families, 10 to 15 percent of women with inherited susceptibility mutations of the BRCA1 gene will never develop breast cancer. Environmental factors and possibly other inherited factors are required as well. In women without a family history of breast cancer, the penetrance of a BRCA1 or BRCA2 mutation is even lower.10 Alleles at other gene loci and similar environments are more likely to be shared by relatives than by people in the general population.
Sensitivity can be estimated by determining the proportion of all known (symptomatic) patients with the disease in whom the test is positive. For direct DNA tests for inherited mutations whose causal role has been established, the mutation is not an effect of the disease. Therefore, determining the sensitivity in symptomatic people is a valid measure of its sensitivity among asymptomatic people. This might not be the case for tests of enzyme activity or metabolite concentration, however. They might be "effects" rather than "causes." Moreover, substances might interfere with their detection. Consequently, validation entails performing the test in healthy individuals. This can be accomplished in pilot screening programs discussed further in chapter 3.
PPV can be estimated by comparing the frequency of positive test results in healthy people younger than the age at which the disease first manifests to their frequency in healthy people who exceed the age by which the disease usually appears. Subtracting the second frequency from the first gives a crude estimate of penetrance. This method does not take into consideration differences in mortality rates from competing causes. A more definitive but time-consuming method is prospective followup of people tested in a pilot study. Having a treatment available that might prevent symptoms of the disease complicates such a study. If all people with positive tests results are treated, it will be impossible to determine whether the failure of the disease to manifest is due to incomplete penetrance or the effects of the intervention. A randomized controlled trial, in which only half of the subjects at risk are treated, can help establish the efficacy of the intervention and the penetrance of the inherited mutation.
Prospective studies can take years. If widespread use of a genetic test is withheld until PPV is fully determined, manufacturers and commercial laboratories could be inhibited from developing tests and, consequently, people denied the benefits that might accrue as a result of being tested. Later in this chapter we discuss solutions to this problem.
Parameters of clinical validity will depend in part on the group or population in which the test will be used. For instance, the frequency of disease-related alleles might differ between ethnic groups, making it difficult if not impossible to extrapolate test sensitivity from one group to another. This is the case for cystic fibrosis and breast cancer in which certain alleles can predominate in one ethnic group or geographical area but not in others.11,12
Penetrance can also differ among ethnic groups. The prevalence of allele frequencies will have a marked effect on PPV; the greater the prevalence, the higher the PPV. Age will also affect allele prevalence; in a population older than the age at which the disease usually causes death, the allele frequency will be lower than in a younger population. For all these reasons, validation studies should be conducted in a group representative of the one in which the test is intended for clinical use.When tests developed for one purpose are used for another, there is no assurance that the sensitivity or PPV will be the same. The maternal serum alpha-fetoprotein (MSAFP) test was formally validated and approved by the Food and Drug Administration (FDA) as a screening test for open fetal neural tube defects. When it was subsequently discovered that a low MSAFP could predict an increased probability of Down syndrome in the fetus, it quickly was used for this purpose without systematic formal validation. The sensitivity and PPV of the MSAFP test for Down syndrome and other chromosome abnormalities are lower than for neural tube defects.13 Data on a particular intended use of a test is needed before that use becomes generally accepted clinical practice.d
The three following criteria help ensure that appropriate data on the clinical validity of genetic tests will be collected during the developmental stages.
(clinical sensitivity, specificity, and predictive value) must be collected under investigative protocols. · Data to establish the clinical validity of genetic tests
· In clinical validation, the study sample must be drawn from a group of subjects representative of the population for whom the test is intended.
·
Formal validation for each intended use of a genetic test is needed.Clinical Utility
The development of tests to predict future disease often precedes the development of interventions to prevent, ameliorate, or cure that disease in those born with genotypes that increase the risk of disease. Even during this therapeutic gap, benefits might accrue from testing as discussed in chapter 1, such as the ability to avoid the conception or birth of an affected child, reduction of uncertainty and, in those with negative results, escape from frequent monitoring for signs of disease or prophylactic surgery and fear of insurance or employment discrimination. In the absence, however, of definitive interventions for improving outcomes in those with positive test results, the benefits will be limited and not everyone will want to be tested. To improve the benefits of testing, efforts must be made as tests are developed to investigate the safety and effectiveness of new interventions. In the absence of such interventions, studies must be mounted to ensure that testing is beneficial and, particularly, does not inflict psychological harm. The balance of benefits to risks will sometimes depend on how the information is presented and who presents it. These issues are candidates for study. The effect of testing on people with negative, as well as positive results, is important to assess. In high-risk families, people with negative results might have assumed they would be affected and are unprepared to cope with a negative result. They might feel guilt for not having the problem afflicting their affected relatives.14 For genetic susceptibility testing, people with negative results might gain the false impression that they have no chance of getting the disease and persist in or undertake unhealthful behaviors possibly to their future detriment. Ways should be sought to present information and explanations to minimize inappropriate or erroneous interpretations (see chapter 4). Learning why people who are offered testing decide not to be tested might also help improve understanding of people's perceptions of genetic testing.
The scientists and laboratories developing genetic tests might not have the expertise to explore a number of issues related to communication and counseling. Collaboration with clinical geneticists, genetic counselors, and psychologists can improve the quality of studies looking into these aspects of test development.
Before a genetic test can be generally accepted in clinical practice, data must be collected to demonstrate the benefits and risks that accrue from both positive and negative results.
ENSURING COMPLIANCE WITH CRITERIA
Because of the length of time it can take to establish the appropriateness of a test for clinical use, it is all the more important to ensure the collection of data on safety and effectiveness in the course of test development. At present, no government policy requires the collection of data on clinical validity and utility for all predictive genetic tests under development. Under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), any laboratory providing tests on which clinical decisions are based must demonstrate the tests' analytical validity to outside surveyors, but CLIA has no provision for review of clinical validity or utility. Under the Medical Device Amendments to the Food, Drug, and Cosmetic Act, the safety and effectiveness or substantial equivalence (to devices marketed prior to passage of the Medical Device Amendments in 1976) of clinical diagnostic testing devices, which include genetic testing devices,e must be demonstrated prior to marketing. FDA considers clinical validity in assessing safety and effectiveness of clinical laboratory testing devices, but generally not data on followup interventions. The FDA's requirements for demonstrating safety and effectiveness are limited to developers who plan to market genetic testing kits.f The FDA has acknowledged to the Task Force that it has the authority to regulate genetic tests marketed as services but is not doing so. (Personal communications from D. Bruce Burlington, M.D. Director, Center for Devices and Radiological Health, FDA, April 3, 1996) Organizations applying for Federal grants to develop genetic tests must submit their research proposals to peer review "study sections." Institutional review boards (IRBs) must also approve protocols submitted to study sections for Federal funding. Many genetic tests, particularly for common disorders, are being developed without Federal funds for research and are not, therefore, subject to peer review. Under FDA regulations, organizations developing new medical devices must have their investigational protocols approved by an IRB. If test results are reported for clinical use and there is no confirmatory test available, the developer must comply with FDA's Investigational Device Exemption regulations. The FDA has not enforced this regulation for developers planning to market tests as services. A number of organizations developing or offering genetic tests, including those who market their own tests (home brews), have never submitted a protocol to an IRB or contacted FDA.
Institutional Review Board (IRB) Review
Considering the structures for external review of research in the U.S. today, the Task Force is of the opinion that IRBs are the most appropriate organizations to consider whether the scientific merit of protocols for the development of genetic tests warrants the risk, however minimal, to subjects participating in the research.
Protocols for the development of genetic tests that can be used predictively must receive the approval of an institutional review board (IRB) when subject identifiers are retained and when the intention is to make the test readily available for clinical use, i.e., to market the test. IRB review should consider the adequacy of the protocol for: (a) the protection of human subjects involved in the study, and (b) the collection of data on analytic and clinical validity, and data on the test's utility for individuals who are tested. IRB review is not needed for minor changes in tests (e.g., detection of additional mutations) as long as the original test was reviewed by an IRB. IRBs may request notification of such changes, however.
Tests under development must be conducted in CLIA-certified laboratories if the results will be reported to patients or their providers.
Health department laboratories or other public agencies developing new genetic tests that satisfy these conditions must also submit protocols to properly-constituted IRBs.
In the early stages of test development, analytical validity and clinical sensitivity can be established using specimens from which identifiers have been removed. (For clinical sensitivity, it need only be known whether the specimen came from someone with disease; identity need not be known.) Using specimens stripped of identifiers prevents contacting subjects. In this case, IRB approval is not needed, although some IRBs might want to know of such studies.15 It would be more problematic to remove identifiers (anonymizing) in an attempt to estimate PPV. Positive test results on specimens from people who were healthy at the time the specimen was collected need to be followed up to see if disease subsequently appeared. Plans to contact the people or examine their medical records require IRB review and approval. Although recontact might be needed to establish PPV, it might not be appropriate to inform people of results. Informing of results would be appropriate only at a stage when the clinical validity of the test has been fairly well established and when some benefit accrues to the subject from knowing the result. Protocols should spell out what subjects will be told when they are invited to participate in the study, if and under what circumstances they will be recontacted and how recontact will be made, and under what circumstances they will be given results.
The Task Force recognizes that the development of genetic tests is an iterative process; methodological changes to improve sensitivity and, perhaps, specificity, will be made. As already indicated, such changes do not require submission of new protocols to an IRB. Changes in the population or group being tested in the developmental stage, or in the purposes of testing should be submitted for IRB review, with appropriate justification, as an amendment to the original protocol.
Is Review of the Scientific Merit of Genetic Test Protocols Within the Purview of IRBs?
Institutional review boards were established to protect human subjects from the risks of participating in research.
g Genetic test development entails a quest for information in order to advance medical practice and clearly falls under the rubric of research. Any research involving humans entails some risk. Even for research in which the risk to subjects is minimal, the risk should not be taken unless the research has scientific merit. OPRR has commented "if a research project is so methodologically flawed that little or no reliable information will result, it is unethical to put subjects at risk or even to inconvenience them through participation in such a study (emphasis added).18( p. 4-1) As part of their duty to protect, IRBs must assess the scientific merit of protocols. Most protocols for the development of genetic tests will have scientific merit if they satisfy the criteria enumerated above. In order to protect human subjects in the development of genetic tests, IRBs must recognize the risks posed by genetic test development and determine that investigators have taken adequate steps to apprise subjects of these risks and reduce the chance of harm from those risks.19,20Improving IRB's Ability to Review Genetic Test Protocols
. The Task Force recognizes that assistance to IRBs in assessing genetic testing protocols would be helpful. After receiving considerable comment, the Task Force rejected creation of a National Genetics Board (NGB) that could review protocols requiring stringent scrutiny or set general guidelines for IRB review and provide consultation to IRBs on request.21 An NGB would add another layer of bureaucracy and further delay approval of research protocols.The Task Force recommends that the Office of Protection of Human Subjects from Research Risks (OPRR) develop guidelines to assist IRBs in reviewing genetic testing protocols. The proposed Secretary's Advisory Committee should work with OPRR to accomplish this task. OPRR and the Advisory Committee should consider how they can be kept apprised of protocols being submitted, in order for them to formulate relevant advice. One possibility is that IRBs submit a one page summary of each genetic testing protocol to OPRR or the group that is developing guidance. The information could include the name of the investigator and his/her institution, the disease for which the test is being developed, intended use, method proposed, and population being studied. Based on these brief reports, the group developing guidance could request protocols for further study but would have no authority to interfere with local IRB review. The protocols would help the group develop general guidance criteria for local IRBs in future reviews.
In developing guidelines for IRBs, OPRR should focus first on tests under development that require stringent scrutiny. The proposed Secretary's Advisory Committee or its designate, in cooperation with OPRR, should establish criteria for stringent scrutiny. In addition to the three criteria mentioned in chapter 1--(1) tests that have the ability to predict future disease in healthy or apparently healthy people; (2) tests that are likely to be used for predictive purposes; and (3) tests for which no independent confirmation is available--others should be considered.
These criteria include: (4) tests likely to have low sensitivity (due to genetic heterogeneity) and low positive predictive value (due to incomplete penetrance); (5) tests for which no intervention is available or proven to be effective in those with positive test results; (6) tests for disorders of high prevalence; (7) tests likely to be used for screening; and (8) tests likely to be used selectively in ethnic groups with higher incidence or prevalence of the disorder.hConflict of Interest
. The Task Force recommends strenuous efforts by all IRBs (commercial and academic) to avoid conflicts of interest, or the appearance of conflicts of interest, when reviewing specific protocols for genetic testing. OPRR should consider more stringent standards for all types of IRBs for avoiding conflict of interest situations. Situations in which a close colleague of the investigator is also the local expert on genetic testing pose a difficult problem for university IRBs. Such colleagues should recuse themselves and, if necessary, the IRB should obtain outside consultation. Another difficult situation arises in small companies in which development of a test is crucial to the company's success. Companies should consider using independent IRBs to avoid the appearance of a conflict of interest.Enforcement
. As previously mentioned, organizations that are developing genetic test kits would be expected to submit their investigative protocols to IRBs. FDA can decline to consider applications containing data from clinical investigations that have not been approved by an IRB. Organizations using Federal research funds for genetic test development are also required to obtain IRB approval. Tests developed without Federal funds, either commercially, in academic clinical laboratories, or in some health departments, are not, at the moment, in legal jeopardy if they do not obtain IRB approval.i Testing organizations should comply voluntarily with obtaining IRB approval of genetic test protocols. Other options the Task Force considered for enforcing the requirement for IRB approval included ensuring that: (1) the FDA use its authority to require all test developers, regardless of whether they plan to market tests as services or kits, to submit protocols to IRBs, (2) third-party payers refuse to reimburse for a genetic test unless the developer can show that it conducted validation/utility studies under an IRB-approved protocol,j (3) clinical laboratory surveyors (see chapter 3) confirm that laboratories have received IRB approval of the new genetic tests they developed, and (4) Congress enacts legislation requiring submission of all research protocols, regardless of support, to an IRB.Data Collection
Investigators given IRB approval for their genetic test protocols have the primary responsibility for data collection under the protocols. To expedite data collection, collaborative efforts will often be needed. For uncommon diseases, a single investigator will seldom have a sufficient number of specimens that contain all or most possible disease-related mutations. Collaboration with investigators who can provide independent sets of specimens or patients increases the likelihood that more mutations will be represented and lends greater statistical confidence to assessments of validity. In assessing tests for susceptibility mutations, having a wider range of patients of various ages obtained from different sources will shorten the time to getting a reliable estimate of PPV. Collaboration will also expedite assessing the safety and effectiveness of interventions in people with positive test results that might be included in protocols to measure test validity.
In other research fields, collaborative research has sometimes been delayed by the necessity of obtaining the approval of each collaborating institution's IRB under current regulations.22 OPRR, with input from the proposed Secretary's Advisory Committee on genetic testing, should streamline the requirements for IRB review of multicenter collaborative protocols for genetic test development in order to reduce costs and get the studies quickly underway. The Task force calls on Federal agencies, particularly NIH and the Centers for Disease Control and Prevention (CDC) to support consortia and other collaborative efforts to facilitate collection of data on the safety and effectiveness of new genetic tests. CDC should play a coordinating role in data gathering and should be allocated sufficient funds for this purpose. In any sharing or pooling of data, confidentiality of the subject source of the data must be strictly maintained. There is, for instance, no reason why a central coordinating agency needs to know the names of subjects with positive or negative test results.
Because it has programs in place, CDC's role is particularly suited to collecting data in healthy populations (e.g., on disease-related allele frequencies). CDC could also establish procedures for tracking healthy individuals with positive test results, as well as those diagnosed with inherited disorders, to learn more about test validity, the natural history of such disorders, and the safety and effectiveness of interventions. The collection of this data should be undertaken in cooperation with test developers, health care providers, and consultants in genetics and other relevant specialties.
CDC could also function as a repository of data submitted to it by organizations competing in the development of a specific test who might not want to collaborate and share data. Respecting proprietary rights, CDC could periodically and confidentially assess the pooled data for validity and utility of the test, providing feedback to the participants on the overall findings.
The Task Force welcomes recent CDC initiatives to expand its population-based surveillance systems in order to provide data on the validity of genetic tests and post-test interventions, and to conduct epidemiologic studies to learn more about test validity, the natural history of genetic disorders, and the safety and effectiveness of interventions. These efforts should be in collaboration with other Federal and State agencies and private organizations.
The Need for Post-market Surveillance
Compliance with all of the criteria for assessment of genetic test validity and utility might be difficult.
It can take years to determine whether a disease will appear in healthy people with positive test results or to establish whether an intervention is safe and effective in preventing or ameliorating the disease in question. The Task Force is concerned that the requirements for prolonged data collection might inhibit test development, especially if commercial firms cannot secure a profit until a test is recognized as being suitable for clinical use.k Adoption of the recommendations in the following section would facilitate rapid introduction with collection of the data necessary for assessing validity and utility.The Task Force recognizes that assessing the validity and utility of some genetic tests will take a long time. When preliminary data indicate a test is likely to have validity and utility, the test should be approved for marketing (see below) but developers must continue to collect data until more definitive answers are obtained. Options for encouraging collection of the requisite data include the following.
(1) Voluntary collection of data by developers after their tests enter clinical use. They would have to develop a reporting mechanism to correlate test results with subsequent occurrence of disease. CDC could coordinate collection of this data from different testing laboratories as discussed above.
(2) Reimbursement for, or coverage of, tests by third-party payers, including government programs, such as Medicare, Medicaid, CHAMPUS, and managed care organizations, during investigative stages in which data are being collected.23
(3) Conditional premarket approval by the FDA of genetic test kits. When FDA considers it likely that the test will prove to make an important contribution to the prevention or management of the disorder, it should grant conditional premarket approval when a developer requests it. (FDA frequently clears or approves products for a limited-indication use with the requirement for postmarket studies or the expectation that claims may be extended as sufficient evidence accumulates.) Tests deemed to require stringent scrutiny should be included. In return for conditional approval, developers could include a profit markup in the price. They could promote the test but would have to indicate that the safety and effectiveness of the test were still under investigation. Informed consent would be needed, but as noted in chapter 1, informed consent for predictive genetic tests is a Task Force principle for many genetic tests in clinical use. Developers of kits would continue to collect and periodically present data to the FDA until such time as the agency gives unconditional approval for marketing. FDA should be required to review the data periodically and decide at each point whether to grant unconditional approval, continue data collection under conditional approval, or revoke conditional approval.
EVIDENCE-BASED ENTRY OF NEW GENETIC TESTS INTO CLINICAL PRACTICE
Although IRBs receive a final report of investigative studies they have approved, they have no responsibility to assess the quality of the data or whether it supports the conclusions of the investigators. Considering the potential widespread use of some genetic tests and their importance, test developers must submit their validation and clinical utility data to internal as well as independent external review. In addition, test developers should provide information to professional organizations and others in order to permit informed decisions about routine use. External review should take place after data have been collected and near the point when developers believe their tests are ready for clinical use not exclusively under investigative protocols. The Task Force recognizes that not all new genetic tests are in need of such review. The proposed Secretary's Advisory Committee should suggest criteria for external review, and recommend means of ensuring that review of tests requiring stringent scrutiny will take place. To accomplish the latter, the cooperation of various government and nongovernment groups to conduct reviews must be secured, as well as funds to support the reviews. Review should take place at the local, as well as national level. A wide range of stakeholders should participate in reviews.
Review panels could become enmeshed in endless debate if they attempt to set cutpoints for sensitivity and PPV; these should vary depending on the particular test, its use, options for treatment, and other factors. Even for a particular test, reasonable people will differ on how much test uncertainty they can tolerate.24,25 It is more important for external reviewers to ensure that the data have been appropriately collected and analyzed than to attempt to set cutpoints. They should also review proposed informational material to make sure the data are interpreted correctly and that test limitations (such as imperfect sensitivity and PPV) are indicated. Review panels could suggest those groups that should consider using the test and those that should not.
The iterative nature of test development makes it likely that methodological improvements will be made in predictive genetic tests. If such changes are made prior to external review, developers can use the data collected before the changes as a "baseline" to demonstrate the improvements, e.g., in test sensitivity. If a test has already been externally reviewed, and the methodological changes alter the target groups, the purposes of testing, or other significant aspects, re-review should be considered by the proposed Secretary's Advisory Committee or other organizations.
Local Review
The first level of local review is by the clinical laboratory that plans to make the test available for clinical use (see chapter 3). In addition, independent local review is also needed, particularly to assess clinical validity and utility. The Task Force strongly suggests that any organization in which tests are developed conduct a structured review of the analytic and clinical validity and utility of new genetic tests before marketing them or otherwise making them available for clinical use. This structured review should be conducted by those not actually involved in developing the test and collecting the data. Some medical centers have standing committees that review tests proposed to be offered in the institution's clinical laboratories that could serve this function. For commercial organizations, a unit within the company, but independent of the laboratory that is actually developing the test, should review the data.
National Review
Current legal requirements that genetic tests be reviewed prior to their clinical use apply only to tests marketed as kits, which require premarket approval by FDA. Even if FDA were to include in its purview genetic tests marketed as services, its review would not address all issues of concern to the Task Force. First, FDA does not generally assess safety and effectiveness of a laboratory test in terms of its ability to improve outcomes of those undergoing testing. Second, FDA generally limits its review to the intended uses of a test claimed by the test's sponsor in its premarket notification. Except when it restricts use of a test to specified purposes, which it has the authority to do, FDA does not exert its power to prevent a test marketed for one intended use to be used for other purposes. This is one reason why the Task Force urges developers to undertake formal validation for each intended use of a genetic test.
To improve FDA perspectives on genetic testing and related issues, the Task Force recommends that FDA bring together consultants on genetic testing either from existing panels or by constructing a new panel to provide guidance to FDA on the classification levels needed for genetic testing devices with single or multiple intended uses. Not all devices may require comparable types of review. In conjunction with the proposed Secretary's Advisory Committee considering stringent scrutiny of genetic tests, these consultants should identify aspects of genetic testing that affect the classification level.
Although no other legally-required mechanisms currently exist, other reviews can have a profound influence on providers' decisions to use, or not use, new medical technologies. Examples are: statements of professional societies, consensus development panels, and ratings by the U.S. Preventive Services Task Force.26 The decision of health insurers on whether a specific genetic test will be included in their benefits or reimbursement packages can also influence use and will be based on the insurers' own reviews (M Schoonmaker, submitted for publication) or other external reviews. A recent consensus development panel on cystic fibrosis carrier screening provides an example of national external review.4
Review organizations could select the tests in the greatest need of review by using the criteria for stringent scrutiny to be developed by the proposed Secretary's Advisory Committee. The reviews would be based primarily on data collected during the test development stage or during the proposed conditional premarket approval stage. Depending on how interest in a test expands, on technological changes, and on other considerations, reviewers could periodically reassess the test as the Preventive Services Task Force does for the interventions it reviews.26
REFERENCES
1. Risch N, Botstein D: A manic depressive history. Nature Genetics 1996;12:351-353.
2. Schwab SG, Albus M, Hallmayer J, et al: Evaluation of a susceptibility gene for schizophrenia on chromosome 6p by multipoint affected sib-pair linkage analysis. Nature Genetics 1995;11:325-327.
3. Lander E, Kruglyak L: Genetic dissection of complex traits: Guidelines for interpreting and reporting linkage results. Nature Genetics 1996;11:241-247.
4. Howell RR, Borecki I, Davidson ME, et al: National Institutes of Health Consensus Development Conference Statement: Genetic testing for cystic fibrosis 1997;in press.
5. Healy B: BRCA genes--Bookmaking, fortunetelling, and medical care. New England Journal of Medicine 1997;336:1448-1449.
6. Friend S, Borresen AL, Brody L, et al: Breast cancer information on the web. Nature Genetics 1995;11:238-239.
7. Morrison-Bogorad M, Phelps C, Buckholtz N: Alzheimer disease research comes of age. The pace accelerates. JAMA 1997;277:837-840.
8. Seshadri S, Drachman DA, Lippa CF: Apolipoprotein E e4 allele and the lifetime risk of Alzheimer's disease. What physicians know, and what they should know. Archives of Neurology 1995;52:1074-1079.
9. Feder JN, Gnirke A, Thomas W, et al: A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nature Genetics 1996;13:399-408.
10. Struewing JP, Hartge P, Wacholder S, et al: The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Askhenazi Jews. New England Journal of Medicine 1997;336:1401-1408.
11. Cutting G: Cystic fibrosis. in Rimoin DL, Connor JM, Pyeritz RE (eds): Principles and Practice of Medical Genetics. London, Churchill Livingstone; 1996.
12. Szabo CI, King M: Invited editorial: Population genetics of BRCA1 and BRCA2. American Journal of Human Genetics 1997;60:1013-1020.
13. American College of Obstetricians and Gynecologists: Maternal serum screening. ACOG Educational Bulletin 1996;228:1-9.
14. Wexler NS: The Tiresias complex: Huntington's disease as a paradign of testing for late-onset disorders. FASEB Journal 1992;6:2820-2835.
15. Clayton EW, Steinberg KK, Khoury MJ, et al: Informed consent for genetic research on stored tissue samples. JAMA 1995;274:1786-1792.
16. The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research: The Belmont Report. Ethical principles and guidelines for the protection of human subjects of research. OPRR Reports April 18, 1979:2-8.
17. Holtzman NA, Andrews LB: Ethical and legal issues in genetic epidemiology. Epidemiologic Reviews 1997;in press.
18. Office of Protection from Research Risks: Protecting Human Research Subjects. Institutional Review Board Guidebook. Washington DC, U.S. Government Printing Office; 1993.
19. Glass KC, Weijer C, Palmour RM, Shapiro SH, Lemmens TM, Lebacqz K: Structuring the review of human genetics protocols: Gene localization and identification studies. IRB 1996;18:1-9.
20. Glass KC, Weijer C, Palmour RM, Lemmens TM, Shapiro SH: Structuring the review of human genetics protocols part II: Diagnostic and screening studies. IRB: A Review of Human Subjects Research 1997;in press.
21. National Institutes of Health: Proposed recommendations of the Task Force on Genetic Testing; Notice of meeting and request for comment. Federal Register 1997;62:4539-4547.
22. Levine RJ: Ethics and epidemiology. in Coughlin SS, Beauchamp TL (eds): New York, Oxford University Press; 1996:257-273.
23. Gleeson S: Blue Cross and Blue Shield Association initiatives in technology assessment. in Gelijns AC, Dawkins HV (eds): Medical Innovation at the Crossroads Vol IV: Adopting New Medical Technology. Washington DC, National Academy Press; 1994:96-100.
24. Tambor ES, Bernhardt BA, Chase GA, et al: Offering cystic fibrosis carrier screening to an HMO population: Factors associated with utilization. American Journal of Human Genetics 1994;55:626-637.
25. Croyle RT, Dutson DS, Tran VT, Sun Y: Need for certainty and interest in genetic testing. Women's Health: Research on Gender, Behavior, and Policy 1995;1:329-339.
26. U.S.Preventive Services Task Force: Guide to Clinical Preventive Services. Alexandria, International Medical Publishing, Inc. 1996.
CHAPTER 3. ENSURING THE QUALITY OF LABORATORIES PERFORMING GENETIC TESTS
Over 500 clinical laboratories in the United States perform chromosomal, biochemical, and/or DNA-based tests for genetic diseases (see appendix 3). These laboratories must comply with regulations under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), which include biennial inspection, some proficiency testing, and requirements of the specialty in which the laboratory is certified. Although clinical cytogenetics is a specialty under CLIA, there is no broader genetics specialty and, consequently, no special requirements for laboratories performing DNA-based and other types of genetic tests. No proficiency testing programs in genetics or cytogenetics are required under CLIA. New York State requires any laboratory performing tests on New York residents (even if those laboratories are outside of New York) to participate in its quality assurance programs in DNA-based and biochemical genetics. These programs involve onsite inspection but not formal proficiency testing.
a A number of organizations have voluntary programs for quality control of genetic tests; they are described later in this chapter. In a survey conducted for the Task Force in early 1995, 11% of biotechnology companies that provide genetic tests and 16% of nonprofit (primarily university-based) molecular (DNA) labs reported that they neither participated in a formal proficiency testing program nor shared samples informally for quality control (see appendix 3). According to the survey, about 15% of laboratories performing clinical DNA-based tests were not registered under CLIA (see chapter 5 for further discussion of this problem).Although the vast majority of laboratories providing genetic tests perform adequately, the Task Force has two concerns. First, even though most laboratories voluntarily participate in quality programs addressed specifically to genetic tests, they are not required to do so. Consequently, providers and consumers have no assurance that every laboratory performs adequately. Occasionally errors are made. Second, that current requirements under CLIA, with which clinical laboratories must comply, are inadequate to ensure the overall quality of genetic testing because they are not specifically designed for genetic tests and because they do not give sufficient emphasis to pre- and post-analytic phases of testing. Voluntary programs are also lacking on this second point.
In this chapter, we first describe the principles that laboratories should follow in adding new genetic tests to their repertoire. We then consider CLIA's framework for laboratory quality and, in view of gaps in CLIA in the area of genetics, other programs for assessing and improving test performance. We then indicate our concerns about ensuring the quality of the pre- and
post-analytic phases of predictive genetic testing. We conclude with brief consideration of the need for a central repository of materials for genetic testing, direct marketing, and international standardization of quality assurance methods.
PRINCIPLES FOR LABORATORIES ADOPTING NEW GENETIC TESTS
No clinical laboratory should offer a genetic test whose clinical validity has not been established, unless it is collecting data on clinical validity under either an IRB-approved protocol or conditional premarket approval agreement with FDA (one of the options presented in chapter 2). The service laboratory should justify and document the basis of decisions to put new tests into service.
In accord with the recommendations in chapter 2, a clinical laboratory that develops a genetic test would have to submit its data on analytical and clinical validity to external review before offering the test for clinical practice.b If the test has been developed elsewhere, clinical laboratories should carefully review evidence for test validity. If external review by professional societies has led to the publication of indications and guidelines for use, laboratories should adhere to them. Regardless of where the test to be adopted was developed, clinical laboratory directors are responsible for ensuring the analytic validity of each genetic test their laboratory intends to offer before they make the test available for use in clinical practice (outside of an investigative protocol). (Methods for assessing analytical validity are summarized in chapter 2.)Before routinely offering genetic tests that have been clinically validated, a laboratory must conduct a pilot phase in which it verifies that all steps in the testing process are operating appropriately.
In establishing the pilot phase, the laboratory should define endpoints, such as number of tests to be performed,c and the procedures to be used to review the findings, including the organizational body that will review them. If the outcome of this review reveals that the laboratory is not as competent as other laboratories in performing the test, or the test does not detect as many people with the genetic alteration as anticipated, the laboratory should not proceed to report patient-specific results without attempting to rectify the problems. If demand is not sufficiently high to be able to maintain a high level of quality, the laboratory should institute special procedures to ensure quality.During the pilot phase, confidence in the analytic validity of the test can be gained by splitting specimens with another laboratory.
d This phase can be used to detect and correct problems in test requisitions, specimen transport, data analysis and transcription, reporting of results, and user satisfaction. It can also be used to establish that laboratory staff are capable of deciding whether each requisition for the test meets established criteria, and the staff is capable of performing the tests and interpreting the results correctly. The pilot phase should employ laboratory practices as similar as possible to those planned when the test becomes routinely available.
CLINICAL LABORATORY IMPROVEMENT AMENDMENTS OF 1988 (CLIA)
A statutory framework for ensuring laboratory quality was laid down by Congress in the Clinical Laboratory Improvement Act of 1967 and greatly expanded in the Clinical Laboratory Improvement Amendments of 1988 (CLIA). Any laboratory performing "examination of materials derived from the human body for the purpose of providing information for the diagnosis, prevention, or treatment of any disease or impairment of, or the assessment of the health of, human beings" must comply with CLIA.2 Implementation of CLIA is the responsibility of the Health Care Financing Administration (HCFA) and the Centers for Disease Control and Prevention (CDC). Under CLIA, these Federal agencies have developed requirements for laboratory quality assurance and control, personnel, patient test-management and, if a proficiency program is not available, interlaboratory comparison of assays. The stringency of these requirements depends on the complexity level and specialty to which tests are assigned. Despite these basic provisions, the Task Force has serious concerns as to whether CLIA adequately assures the quality of genetic tests in clinical use.
Complexity Ratings
CDC assigns a complexity level to a test according to predetermined criteria. Simple tests are categorized "waived." The remainder are assigned ratings of either "moderate" or "high" complexity. Laboratories performing high complexity tests have more stringent personnel and quality control requirements.
Over 17,000 clinical laboratory tests have been assigned a complexity level.
e Any test for which CDC has not determined test complexity is considered to be high complexity by default.f Any home-brew method, or change in procedure that can affect laboratory performance (sensitivity, specificity, accuracy, precision) falls under the high complexity category until it is rated differently by CDC. Under the rating scheme, a genetic test that can be used predictively might receive a rating of moderate complexity despite the importance of ensuring that the provider and the patient understand the uncertainty of the prediction and the implications for decision-making. Both the creatine phosphokinase test, which can be used as a screening test for Duchenne muscular dystrophy, and alpha-fetoprotein (AFP), which is used as a predictive prenatal test for neural tube defects and Down syndrome, are rated as moderate complexity. Despite multiple uses, a test method gets only one rating based on the seven criteria (see box entitled Complexity Determinations under CLIA) that reflect the complexity of performing the test. All cytogenetic tests are rated high complexity, which seems appropriate. The Task Force recommends that tests that can be used for purposes of predicting future disease be given a rating of high complexity.CLIA Specialties
Laboratories performing tests of moderate or high complexity must also conform to the
requirements of the specialties to which tests are assigned. Laboratories can perform tests only in specialties for which they are certified. Although there is a cytogenetics specialty, there is no genetics specialty.
g The specialty categories under CLIA are based on traditional laboratory practice; each specialty tends to involve somewhat similar technologies, although this is not the case in all instances. Each analyte is assigned to only one specialty.Establishing a specialty for genetics presents a number of problems. For example, specialty designations are administratively linked to Medicare payment specialty designations, and any changes in specialty designations must take this into account. In addition, genetic tests use a wide variety of technologies, some of which are used in other (non-genetic) types of tests. For instance, DNA is the analyte in some tests for predicting genetic susceptibility and also in some tests for infectious agents. Sometimes the same test is used for purposes of genetic prediction (in healthy individuals), genetic diagnosis (in individuals with symptoms), and non-genetic diagnosis or prognosis. For instance, the creatine phosphokinase assay can be used to screen for carriers of muscular dystrophy and affected infants, but it is also used in the diagnosis of myocardial infarction. Despite these problems a genetics specialty is needed.
The Task Force welcomes the intention of CDC to create a genetics subcommittee of the Clinical Laboratory Improvement Advisory Committee (CLIAC), which advises on policies under CLIA. The Task Force urges this subcommittee to consider the creation of a specialty of genetics that would encompass all predictive genetic tests that satisfy criteria for stringent scrutiny. If a specialty of genetics is not feasible, the subcommittee should consider a specialty or subspecialty of molecular genetics for DNA/RNA-based tests. In the latter case, it must then address how to ensure the quality of laboratories performing nonDNA/RNA genetic tests. Although DNA-based tests will comprise the largest proportion of predictive tests, for disorders with great allelic diversity, gene product tests might have greater sensitivity than DNA-based tests, at least until technologies that can detect a large proportion of all possible mutations become applicable to clinical testing. The subcommittee should also consider assigning tests that have widely different uses to more than one specialty. This will facilitate assigning separate billing and reimbursement codes for each use of a genetic test when the uses are vastly different.
LABORATORY PERSONNEL
Personnel requirements under CLIA, particularly at the level of laboratory director, depend on the specialty and complexity categories to which tests or analytes are assigned. Without a genetics specialty, genetic tests fall into other specialties for which requiring special training in genetics would be superfluous for many of the other tests in those specialties.
Laboratory Director
Under CLIA, a laboratory director must possess a current license as a laboratory director in the state in which the laboratory is located and be either (1) a pathologist; (2) a physician licensed to practice medicine or osteopathy; or (3) a board-certified doctoral scientist (Ph.D.).
h The Task Force recommends that for laboratories performing high complexity tests in the proposed specialty of molecular genetics, as well as in biochemical genetics and cytogenetics, personnel serving as directors or technical supervisors must have formal training in human and medical genetics, as documented by holding certification from an organization that assesses knowledge of human and medical genetics as part of its certification process, such as the American Board of Medical Genetics.Testing Personnel
CLIA imposes minimal academic qualification requirements for testing personnel.
i This is reasonable in the area of genetics because most current medical technology training programs include little, if any, exposure to genetics or molecular biology. Several formal training programs for cytogenetics technical staff are available, but there are very few certificate- or diploma-track genetics training programs for technicians or technologists in the U.S. Consequently, most technicians in molecular genetic testing laboratories are trained on the job. Broad backgrounds in genetics are unlikely, as is a familiarity with specialized methodologies involved in molecular genetics testing. Training programs for laboratory technicians/technologists need more human and medical genetics content than are currently available in the U.S.The College of American Pathologists (CAP) specifies a B.S. degree or equivalent in the biological sciences for technologists engaged in genetic testing. Neither CAP nor the American College of Medical Genetics (ACMG) requires personnel to be licensed medical technologists but some States require it. Many States offer a special licensure for cytogenetics technicians; this is a desirable attribute where available. California is presently trying to develop a similar licensing mechanism for molecular genetics technicians. The National Certification Agency is working with the Association of Genetic Technologists to develop certification in genetics. Licensing of technologists performing genetic tests can then be linked to certification. Most clinical molecular genetics laboratories employ technicians with a molecular biology research background.
Biochemical genetic techniques resemble those used in other, more routine, areas of clinical chemistry. For this area, therefore, Federal, State, and professional requirements for clinical chemistry laboratory personnel are sufficient, as long as the technologists work under a director who is a certified biochemical geneticist.
MONITORING LABORATORY PERFORMANCE
Because laboratories provide services to providers and patients in many States it is clearly more desirable to have a rigorous Federal standard for certification or accreditation than fifty different State standards. Moreover, interstate genetic testing is unavoidable when only one or a few laboratories in the country provide tests.
A national accreditation program for laboratories performing genetic tests, which includes proficiency testing and on-site inspection, is needed to promote standardization across the country. Such an accreditation program can occur more readily if a genetics specialty were established under CLIA. Until such time as a genetics specialty is established under CLIA, laboratories performing DNA/RNA-based tests for predictive purposes should choose to voluntarily participate in the CAP molecular pathology program including the CAP/ACMG molecular genetics proficiency testing program. Laboratories performing genetic tests on analytes not covered in the CAP/ACMG program, such as Tay-Sachs carrier screening and newborn screening, should participate in the available proficiency programs.Proficiency Testing
Proficiency testing (PT) is mandated by CLIA to externally evaluate the quality of a laboratory's performance. For PT, a laboratory is provided with specimens whose composition of an analyte is known to the supplier but not to the recipient laboratories. They are expected to analyze the specimen the same way they would a patient's specimen. Each laboratory performing moderate or high complexity tests is required to enroll in an approved PT program for all specialties/subspecialties, analytes, or tests for which the laboratory is certified and for which a PT program has been recognized by HCFA. Any laboratory that fails a proficiency test must take corrective action.
j HCFA takes an educational approach to PT and works with the laboratories that have problems to help improve performance. Sanctions can be applied to those laboratories repeatedly unable to perform satisfactorily. These include suspension of the CLIA certificate to perform that test or specialty. If its certificate is suspended, the laboratory is not eligible for Medicare/Medicaid reimbursement, since such reimbursement requires a CLIA license with no restrictions.So far, the Department of Health and Human Services has approved 19 PT programs under CLIA. It has not approved proficiency testing programs for genetic tests because such tests do not measure regulated analytes for PT purposes as currently listed in the regulations. New York State and a few regions have cytogenetics PT programs. CAP and ACMG jointly administer PT in cytogenetics, fluorescent in situ hybridization, biochemical genetics, and molecular genetics. In collaboration with the Foundation for Blood Research (FBR), CAP has a PT program for prenatal screening of neural tube defects and Down syndrome. CDC has a PT program for newborn screening tests, including hemoglobinopathies. PT is also available for laboratories worldwide performing Tay-Sachs screening. Responding to a survey conducted for the Task Force in July 1997, CAP, FBR, CDC, and the International Tay-Sachs program reported that most laboratories known to them were participating in their respective programs.
kAlthough genetic tests do not appear on the list of regulated analytes for PT purposes under CLIA, laboratories must establish the accuracy and reliability of a test by methods of their own choosing. This can include participation in one of the voluntary PT programs. As the PT programs mentioned above are not approved by CLIA, no laboratory is obliged to use them and can establish accuracy and reliability by another method, although it must make the data available for onsite inspection under CLIA (see below). If they do participate and do not perform adequately, laboratories will usually improve performance. If, however, they continue to fail to meet PT criteria, they are not obliged to stop testing as participation is voluntary. A few laboratories participating in the PT programs recently surveyed do not always correctly analyze all PT specimens. According to the Tay-Sachs program, one or two per year do not improve and usually stop testing.
Information collected in conjunction with PT sometimes reveals outliers among laboratories. For instance, a survey conducted by the FBR/CAP prenatal screening PT program found a few laboratories that did not follow established criteria in accepting specimens.
Participation in well-established proficiency testing programs for genetic tests must be required under CLIA once a genetics specialty is established. When no relevant proficiency testing programs exist, laboratories must, whenever possible, participate in inter-laboratory comparison programs and help develop them if none exist in their particular area of testing.
Proficiency testing programs should be broadly based since the number of genetic disorders is very large and the analytical approaches to testing are numerous. It is unlikely that proficiency challenges will ever be constructed for every rare disease or every rare mutation in common diseases for which a given laboratory might test.
Because of the similarity of techniques used in biochemical genetics, proficiency in these techniques applied to one or a few analytes is a reasonably good indicator of proficiency in other uses of the technique. CAP/ACMG is expanding the PT offering in molecular genetics to a greater number of disorders in order to get more complete demonstrations of proficiency.Onsite Inspection
All CLIA-certified laboratories
are routinely inspected on a two-year survey cyclel by one of three types of organizations: (1) HCFA regional offices and State agencies; (2) private non-profit organizations that have applied for and received deemed status because they provide reasonable assurance that the laboratories they accredit, which enables the laboratory to obtain a CLIA certificate, meet the conditions required by Federal law and regulation;m (3) State-exempt licensure programs. States that have programs that license laboratories and provide HCFA with reasonable assurance that their criteria are equivalent to or more stringent than those specified under CLIA can apply for exempt status. So far New York, Oregon, and Washington (state) have exempt status. California, Florida, and Georgia are under review (as of July 1997). Regardless of the organization under whose auspices inspections are conducted, the surveyors are laboratory professionals who are trained to determine compliance with CLIA regulations (or a program that is determined to be equal to or more stringent than CLIA). Even though genetics is not a specialty, surveyors are expected to examine the quality of genetic tests. This should include inspection of the records of how the laboratory performed on genetic PT programs in which it participated voluntarily. It is not clear, however, that all CLIA surveyors currently are sufficiently knowledgeable to assess the performance of molecular genetics laboratories.nCAP has deemed status to conduct inspections in several specialties, but since genetics is not a specialty under CLIA, the CAP program does not have deemed status in genetics. In the CAP genetics program, laboratories who voluntarily (and for a fee) participate in the program are inspected. The surveyors use a checklist covering all aspects of quality assurance and quality control, from specimen accessioning to final sign-out. Compliance with some items on the checklist is optional; for others, compliance is mandatory.
o Following inspection, the laboratory receives a written report and is expected to respond to CAP in writing regarding correction of any deficiencies in the mandatory categories. In areas in which it does not have deemed status, such as genetics, CAP has no authority to grant accreditation for CLIA purposes.
Making Laboratory Performance Assessments Public
HCFA annually publishes a list ("Laboratory Registry") that identifies all poor performance laboratories, the reason enforcement actions were taken and type of enforcement, and the name of the laboratory director. The Registry is available to the public upon request, and will soon be accessible on the Internet at http://www.hcfa.gov. Survey findings are also available through the Freedom of Information Act, once the laboratory has the opportunity to respond with its Plan of Action. CAP reports PT results for regulated analytes (i.e., those for which CLIA requires PT) to HCFA. It does not report PT results directly to the public because it maintains that PT alone is insufficient to demonstrate laboratory quality. CAP does make accreditation status available through its toll-free hotline (1-800-LAB-5678), and a CAP-published list of accredited laboratories.p As CAP is not deemed to accredit in areas of genetics, it does not make the results of its assessments of genetic test performance public.
The Task Force recommends that CAP/ACMG periodically publish, and make available to the public, a list of laboratories performing genetic tests satisfactorily under its voluntary program
. Other PT programs should also publish the names of laboratories performing satisfactorily if they do not already do so. Until then, publication of results in voluntary proficiency and other quality assurance programs enable providers and consumers to select approved laboratories and also serve as an incentive for laboratories to participate in the CAP/ACMG quality assessment program. The information on laboratories performing satisfactorily should be readily accessible to consumers and providers.P
ublishing the names of laboratories performing satisfactorily would advise users that labs not appearing on the list have either not submitted to external review or have not performed adequately. Directories of laboratories providing genetic tests (e.g.. HELIX--see chapter 5) should also publish information on listed laboratories' satisfactory participation in PT and other quality control programs specific for genetic tests. The Association for Molecular Pathology publishes information on the quality of laboratories, and the National Organization for Rare Diseases and the Alliance of Genetic Support Groups make it publicly available. Managed care organizations and other third-party payers should limit reimbursement for genetic tests to the laboratories on published lists of those satisfactorily performing genetic tests. Implementation of this recommendation is especially important as more managed care organizations move to restrict access to laboratory services for their members to a single laboratory with whom each organization contracts. Such a laboratory might not have participated or performed satisfactorily in a quality control program.
A CENTRAL REPOSITORY OF CELL LINES AND DNA
Making cell lines or DNA containing disease-related mutations available to many laboratories would be useful in the validation of new tests, calibration, standardization, and quality control. To accomplish this, appropriate specimens from patients, carriers, and controls should be available through a centralized repository in order to facilitate their availability to aid in analytical validation, improving quality, and other needs. Resources such as the National Institute of General Medical Sciences' Human Genetic Mutant Cell Repository (housed at the Coriell Institute for Medical Research) and the American Type Culture Collection should be utilized.
It should be impossible to trace samples in a repository to the individuals from whom they were obtained. The samples should not be used for any purpose from which a profit could be derived, such as the sale of unusual probes. A central repository of analytes for standardizing biochemical and other types of tests, including those used for screening, is also needed. Some mechanism for ensuring the composition and concentration of these standards, such as FDA review, is needed.
THE IMPORTANCE OF THE PRE- AND POST-ANALYTIC PHASES OF TESTING
In the
pre-analytic phase, laboratories sometimes give information about the test to providers and consumers. Informed consent can be obtained, and data are requested from those to be tested. In the post-analytic phase, test results are given to the provider and patient, often with an interpretation. Genetic counseling services can be provided or arranged by laboratories, but are the responsibility of the referring provider.Pre-analytic Phase
The Task Force is concerned about the quality of information made available to providers and consumers who are considering testing. Some materials haveserious omissions that impair the ability of providers and consumers to make informed decisions about testing. In a comparison of four different brochures made available by organizations offering testing for genetic susceptibility to breast cancer, the Task Force found striking discrepancies. Physicians or consumers reading one brochure might, as a result, make a different decision than if they read another organization's brochure. It is the responsibility of health care providers, not the clinical laboratory, to provide information to the individual offered or considering testing, but material made available by laboratories is often used. The completeness and accuracy of this material is, therefore, extremely important.
Obtaining informed consent helps ensure that the person voluntarily agrees to testing and has some understanding of the reasons for testing. Informed consent is appropriate for predictive genetic tests, particularly those for which stringent scrutiny is needed. The Task Force is of the opinion that laboratories should obtain documentation of informed consent when appropriate and should not perform an analysis if documentation is lacking. The most rigorous documentation is for the laboratory to be sent a signed copy of the patient's consent. It is less rigorous to ask the ordering physician to check a box on the laboratory requisition indicating that consent has been obtained.
Because of the complexities of assessment and interpretation, requisitions for many genetic tests require more intake information than those for virtually any other clinical laboratory procedure. In addition to routine information, genetic test requests often must include the reason for requesting the test, any relevant clinical or laboratory information, the person's age and ethnicity, and notation of family history of the disorder in question (along with a full pedigree for tests involving linkage analysis). If information that is critical to the performance or the interpretation of the test cannot be obtained, or if the information that is provided suggests that the patient is not an appropriate candidate for testing, the physician must be contacted. There is consensus, for instance, that minor children should not be tested for adult-onset disease for which no diagnostic or therapeutic interventions are needed before adulthood (see chapter 1). Yet some laboratories report testing children (see chapter 4 and appendix 3). Most authorities agree that healthy women without a family history of breast cancer should not be tested for inherited susceptibility mutations for breast cancer except under investigative protocols to gather data on the penetrance of these mutations, and that women with a family history of the disease should only be tested if an inherited susceptibility mutation is found in an affected relative.5-7 Consequently, laboratories must ascertain the presence of a family history before accepting a specimen. At least one laboratory is offering testing to Ashkenazi Jewish women without a family history.8 In general, laboratory personnel must be competent to recognize what information is needed and what the criteria are for accepting specimens. When in doubt, they must communicate with the ordering provider.
Post-analytic Phase
Increasingly, genetic tests will be requested by providers without much or any training in genetics. (Recommendations on ensuring provider competence appear in chapter 4.) Accurate and comprehensible interpretation of genetic test results by the clinical laboratories is critical to ensure that the provider understands the implications and can explain them to the persons who were tested.
Genetic test results must be written by the laboratory in a form that is understandable to the non-geneticist health care provider. The quality of laboratories' written interpretations of genetic test results should be included in the overall assessment of laboratories providing genetic tests.Some laboratories also make genetic counselors available to discuss results with physicians. If testing of other relatives is an option, a potential conflict of interest arises as the counselor might want to promote additional business.
Ensuring the Quality of Pre- and Post-analytic Phases
One way of improving laboratory performance is to have more rigorous standards with which laboratories must comply. T
he Task Force is of the opinion that not enough emphasis is placed on the pre- and post-analytic phases in CAP's molecular pathology and special chemistry programs. The Task Force recommends that CAP and ACMG seek advice and input from consumer groups such as the Alliance of Genetic Support Groups, as well as from the National Society of Genetic Counselors (NSGC), on educational, psychological, and counseling issues in pre- and post-analytic components of genetic testing that are of direct concern to consumers.Under CLIA, the rating system used to establish the complexity of tests does not give sufficient weight to these phases (see box entitled Complexity Determinations under CLIA). CDC should consider how the pre- and post-analytic phases of predictive genetic testing can be given greater weight in CLIA standards and regulations.
DIRECT MARKETING OF GENETIC TESTS TO THE PUBLIC
Many clinical laboratories advertise the availability of tests directly to the public (see appendix 4). Great care must be taken that information on genetic tests presented directly to the public is accurate and includes risks and limitations, as well as benefits.
The informational material should be sensitive to the knowledge level of the general public. In addition to describing the benefits and risks of the genetic test(s), including discrimination issues and the potential emotional impact on individuals and family members, the material should describe those for whom testing is appropriate (e.g., couples planning to have children for carrier tests, and individuals with a family history of a late-onset disorder for which genetic predispositions can be detected), and should emphasize that all genetic testing is voluntary, often requiring informed consent. Consumers should discuss testing options with a health care provider competent in genetics prior to having specimens collected for analysis.The Task Force is concerned that no mechanism exists for the review of the accuracy of informational material on genetic tests made available either to providers or consumers, except for the labeling materials on kits that must be reviewed by FDA in premarket applications. As already noted, most genetic tests are marketed as services, not kits. Although complaints concerning inaccurate information can be made to FDA, the Federal Trade Commission, the Consumer Product Safety Commission, or the consumer protection divisions in the offices of most States' Attorneys General, harm could be done from exaggerated claims before complaints are filed or acted on. The external review of tests before they enter clinical use (see chapter 2) should include examination of proposed informational material.
In accord with laws in most States, clinical laboratories in the U.S. require that specimens for the vast majority of tests come from a physician or are reported to a physician. A few laboratories accept specimens for predictive genetic testing directly from consumers without the intervention of their own physician. In such cases, a physician affiliated with the testing laboratory, who is a specialist but may be previously unknown to the patient, can order the test. As DNA can be isolated and amplified from cells in saliva or scraped from the buccal mucosa, it is possible for lay people to collect their own specimens. FDA has the authority to regulate this practice if the laboratory supplies or requires use of a specially designated collection device or container to send specimens from the person's home to the laboratory. The Task Force discourages advertising or marketing of predictive genetic tests to the public.
INTERNATIONAL HARMONIZATION
At present, no mechanism exists to create international standards of laboratory quality and proficiency for genetic tests. Current United States regulations require any foreign laboratories performing clinical laboratory tests on U.S. residents to hold a CLIA certificate even if their nation's laboratory standards are more stringent than those of CLIA. The Task Force recommends that efforts should be made to harmonize international laboratory standards to ensure the highest possible laboratory quality for genetic tests. A proposed European Union Directive on "In Vitro Diagnostic Medical Devices," with which FDA is cooperating, will harmonize the situation for assessing medical devices including genetic test kits and reagents. This Directive, however, does not extend to tests provided as services, similar to the situation in the U.S.
REFERENCES
1. Kaback M, Lim-Steele J, Dabholkar D, Brown D, Levy N, Zeiger K: Tay-Sachs disease--carrier screening, prenatal diagnosis, and the molecular era. An international perspective, 1970 to 1993. The International TSD Data Collection Network. JAMA 1993;207:2307-2315.
2. Bogdanich W: False negative. Medical labs, trusted as largely error-free, are far from infallible. Wall Street Journal Feb. 2,1987:1.
3. Public Law 100-578: Clinical Laboratory Improvement Amendments of 1988. 1988;42 USC 263a.
4. Palomaki GE, Knight GJ, McCarthy JE, Haddow JE, Donhowe JM: Maternal serum screening for Down syndrome in the United States: A 1995 survey. American Journal of Obstetrics and Gynecology 1997;176:1046-1051.
5. Burke W, Kahn MJE, Garber JE, Collins FS: "First Do No Harm" applies to cancer susceptibility testing too. Cancer Journal from Scientific American 1996;2:250-252.
6. Weber B: Breast cancer susceptibility genes: Current challenges and future promises. Annals of Internal Medicine 1996;124:1088-1090.
7. Blue Cross and Blue Shield Association Technology Evaluation Center (TEC): Executive Summary of TEC Assessment on Genetic Testing for inherited BRCA1 or BRCA2 mutations. 1997.
8. Schulman JD, Stern HJ: Genetic predisposition testing for breast cancer. Cancer Journal from Scientific American 1997;2:244-249.
CHAPTER 4. IMPROVING PROVIDERS' UNDERSTANDINGS OF GENETIC TESTING
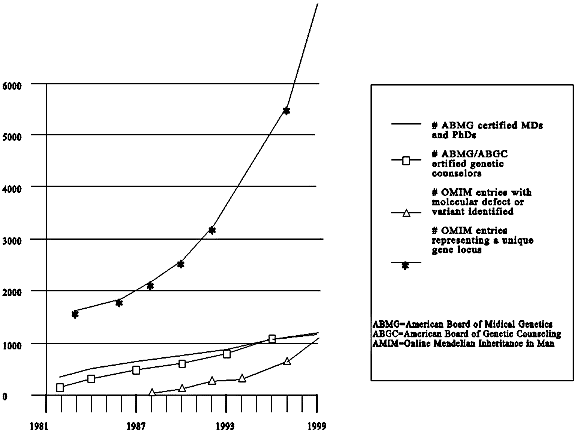
The increase in the number of disease-related genes that scientists have identified in recent years, particularly those in which inherited mutations increase susceptibility to common disorders, has engendered expectations that health care will be improved. The rate of increase of health care professionals trained and board-certified in medical genetics or genetic counseling has not kept pace with the rate of increase of genetic discovery and of potential demand for genetic tests. Although genetic professionals currently in practice or in training could meet a small increase in demand for genetic testing and counseling, their supply is insufficient to cope with even a doubling of the demand. Some commentators maintain that population carrier screening for just one condition, cystic fibrosis, would swamp the system.1 Thus, if the demand for genetic testing increases, and the supply of
genetics providers does not keep pace, other health care professionals will have to play a role, or new models of testing will have to be devised if the demands are to be met. In this chapter, we first delineate a role for non-genetic health care professionals in eliciting genetic risks and providing genetic tests. We then turn to the obstacles of having non-geneticists provide these services. We next consider policies for overcoming the obstacles and, finally, other models for providing genetic services.
A ROLE FOR NON-GENETIC HEALTH CARE PROFESSIONALS
In addition to the paucity of genetic specialists relative to the potential demand for genetic testing, there are other reasons why other professionals should be involved in genetic testing. First, few people have sufficient understanding of genetics to recognize whether or not they or their children are at increased risk of inherited disease. Therefore, health care professionals who provide care to most people have a responsibility to determine whether a high-risk situation is present. With the rise in managed care in the United States, these professionals are increasingly primary care providers who provide first-contact and continuing care and who may serve as gatekeepers for access to other specialists. Nevertheless, in the United States, many people can bypass primary care providers and seek care directly from specialists. Even when aware that a problem that concerns them might have a genetic origin, they are more likely to seek the care of the specialist who manages the problem when it becomes overt than the care of a geneticist. For instance, people concerned about an inherited susceptibility to cancer will go to an oncologist or surgeon more often than to a geneticist, and pregnant women concerned about birth defects or inherited disorders will ask their obstetrician instead of a geneticist. Consequently, non-genetic specialists, as well as primary care providers, become the gateway to genetic testing.
Second, primary care and other providers that people visit periodically are in an excellent position to elicit risk information. One important source of information about genetic risks is family history. When people receive their care from one source over a period of years, as is the ideal primary care situation, the provider is more likely to learn about family history as relatives become ill (whether they are in the provider's care or not) and, possibly, about other situations that raise the risks of genetic disease. If the source remains constant but the providers change, a single medical record used by all of the patient's providers gives the current provider an opportunity to recognize risk factors, if the record is adequate. This advantage is lost when people change their source of care (at least until a universal medical record that people keep with them, such as a "smart card", is developed). Each new provider, including specialists, must attempt to ferret risk factors, including family history, again. The few studies that have been done show that family history, as elicited directly from people, does not always accurately reflect what medical records of relatives contain.2-4 Despite their skill and expertise, genetic specialists who see a person only once, as is often the case in prenatal care, might not be able to elicit as complete a picture of risk factors for genetic disease as the primary care provider who sees the person repeatedly. Moreover, without recognition of genetic risk factors by primary care providers or other non-genetic health care providers, many people will never get to a genetic specialist.
Eliciting Risks of Genetic Disease in Healthy People
Family History. Although family history is an important source of information about risks of future genetic disease, it has limitations. We have already mentioned the problem of reliability. More importantly, its yield will depend on the mode of inheritance of the diseases of concern. It is most useful when diseases are inherited in a dominant or X-linked fashion. Some diseases inherited in these ways will, however, arise by new mutation and the family history will be negative. Eliciting a history of frequently-recurring common diseases that do not follow Mendelian inheritance might indicate the presence of inherited susceptibility, such as those for breast and colon cancer, or of polymorphisms that have been associated with disease. The family history is less likely to be informative for autosomal recessive diseases in which each parent of an affected child is an asymptomatic carrier. Eliciting a history of consanguinity in the parents points to an increased risk of autosomal recessive diseases in their children; the parents might each have inherited the same disease-related alleles from a common ancestor. This also explains why some autosomal diseases are higher in certain ethnic groups in the absence of consanguinity. Thus eliciting a person's ethnicity also becomes important. When people have many children, it is more likely that the family history will be positive for a recessive disease; on average, one out of four children will be affected. Adoption, the use of artificial insemination by donor sperm and multiple sexual partners, as well as people's greater mobility (removing them from the nuclear family), increase the difficulty in eliciting an informative family history. One systematic method of collecting family history data, and also establishing whether consanguinity is present, is the construction of a pedigree.
Past History. In view of the limitation of family history and ethnic origin, the health care provider must look for other ways of determining genetic risk factors for future disease. Some risk factors can also be elicited by interview. These include (1) the age of a pregnant woman; as maternal age increases, particularly over 35 years, the risk of Down syndrome and other chromosomal abnormalities in her fetus increases, and (2) past or present exposure to an environment that is more likely to result in disease in those with genetic predispositions, such as intake of fava beans or anti-malarial drugs in people with glucose-6-phosphate dehydrogenase deficiency, which has a higher frequency in people of African, Asian, and Mediterranean origin.
Genetic Testing. Finally, genetic testing can be used to elicit risks of future genetic disease. If the person's history is unrevealing and if the disease is a serious one that can be avoided by reproductive options, prevented, or more effectively treated by intervention in its presymptomatic stages than after symptoms appear, then population-wide screening can reduce the burden of the disease if it is utilized by many in the population. In the absence of an affected family member, carriers for autosomal recessive disease can be detected by genetic screening. Genetic testing can also confirm the presence of specific disease-related alleles in people with positive histories, pointing the way to specific interventions. Testing and screening should only be undertaken in clinical practice when the conditions for testing described in chapter 2 have been satisfied.
The question can be asked, why not simply screen everyone for disease-related alleles and bypass the family and past history?
First, relatively few predictive tests applied on a population basis meet the criteria of validity and utility described in chapter 2. Second, even if they did, it would be extremely costly to test everyone. As the cost of the technology is reduced, this reason becomes less important. Third, the process of offering predictive genetic screening takes time. In accord with the principles of autonomy presented in chapter 1, people must be informed of the benefits and risks of screening and given an opportunity to decline it. Although this might be accomplished simply by brochures and other audio-visual aids, the effectiveness of these methods has not yet been established. Unless and until they are, providers will have to spend time explaining screening to potential users. Fourth, when the results come back, they have to be interpreted. As discussed in chapter 2, many genetic tests are not perfect predictors. The probability that disease will occur when the test result is negative, or that disease will not occur when the result is positive, both of which will be greater when populations rather than at-risk individuals are tested, must be explained.The Role of Non-genetic Health Care Providers
With proper training and adequate knowledge of test validity, disease and mutation frequencies in the ethnic groups to whom they provide care, primary care providers and other non-genetic specialists can and should be the ones to offer predictive genetic tests to at-risk individuals. In some circumstances, for instance, when the family history is complicated or the symptomatology in relatives does not point to a clear diagnosis, referral to a genetic specialist is appropriate before offering testing. Unless there are other means of providing screening, such as through hospitals (for newborn screening) or public health facilities (see section on Other Models later in this chapter), non-genetic providers will almost always be involved in offering genetic screening, as well as testing. The role of non-genetic providers in interpreting test results is complex. The interpretation of positive results will often depend on further elicitation of risks, including family history. The options available to reduce risks will also have to be considered. Positive results can have implications for future children. Often they will also be of importance to other relatives with whom the person tested should be encouraged to communicate. For tests with imperfect sensitivity and those for susceptibility to common disorders, negative results do not eliminate the chance of future disease. A test's sensitivity and predictive value can also vary by ethnic group (e.g., the sensitivity of current CF carrier tests is much higher in Caucasians than in African or Asian Americans). Providers must be aware of these and other considerations in interpreting test results, and be capable of communicating risk information and its implications to those who are tested or their parents or guardians. Consultation with geneticists and/or genetic counselors might be appropriate.
OBSTACLES TO THE INVOLVEMENT OF NON-GENETIC PROFESSIONALS
Despite the advantages of non-genetic providers being the gateway to genetic testing, there are drawbacks. One is the limited knowledge of genetics and genetic tests of some non-geneticist providers. In a 1991 survey of physicians selected at random from ten states, non-genetic, non-academic physicians in five specialties (family practice, internal medicine, obstetrics-gynecology, pediatrics, and psychiatry) were able to correctly answer an average of 73.1% of questions deemed important by a panel of non-genetic providers who helped develop the questionnaire. Physicians who graduated from medical school between 1971 and 1985 scored significantly higher than those who graduated between 1950 and 1970. Having a genetics course in medical school was significantly associated with higher scores but was not as important a predictor as the year of graduation. Physicians in specialties that had been exposed to genetic problems in their practices (family physicians who delivered babies, pediatricians, and obstetrician-gynecologists) had significantly higher scores than physicians in the other specialties. Over one-third of family physicians who did not deliver babies, internists, and psychiatrists had scores of 65% correct or lower.6
In a 1996 survey on testing for genetic susceptibility to cancer, Burke and Press found that of the first 124 primary care physicians to respond, over 20% had not heard of a test for a genetic predisposition to breast cancer. (N. Press, personal communication)
Another drawback is the tendency of non-geneticist providers to be directive in situations in which reproductive options to avoid the conception or birth of an infant with a serious disorder are considered.7-11 Primary care providers occasionally report that they will not offer a prenatal test to a patient who they are confident would not be interested in testing.8 Whether the provider, even one with a continuing relationship with the patient, really does know the patient's attitudes on this subject and, if so, is justified in withholding information, is debatable. Recently, it has been recognized that nondirectiveness might not be achievable and might not be something that patients always want.12-15 Nevertheless, because of past efforts to deny people the opportunity to reproduce because they possessed presumably heritable traits,16,17 and the need to respect personal autonomy in reproductive matters, efforts to steer people toward a particular reproductive decision are undesirable (see chapter 1).
When safe, effective, and widely acceptable interventions are available for people with positive predictive test results, the role of nondirectiveness is much less of an issue. When interventions are not of proven safety and effectiveness, people should be told that is the case and should decide for themselves whether they want testing and, if they do and subsequently have a positive test result, whether they want the unproven intervention.
It is not clear that primary care providers could devote the time that informing patients about risks and benefits of genetic tests often entails. The average time spent counseling new patients in genetics or prenatal clinics exceeds 1 hour.18 The median time of counseling for molecular genetic testing is 1 hour, not counting preparation (record review) or clerical and administrative time.19
POLICIES FOR IMPROVING THE ABILITY OF NON-GENETIC HEALTH CARE PROFESSIONALS TO BE INVOLVED IN GENETIC TESTING
The Task Force considered a number of strategies, both long and short-term, for improving the ability of non-genetic health care professionals to provide genetic services safely and effectively.
Greater Public Knowledge of Genetics
A knowledge base on genetics and genetic testing should be developed for the general public. Without a sound knowledge base, informed decisions are impossible and claims of autonomy and informed consent suspect. People who are more knowledgeable will grasp more readily the issues raised by providers when they offer tests. This could diminish the time needed for education and counseling without reducing consideration of the implications of testing. Policies for improving public understanding of genetics and genetic tests are beyond the scope of the Task Force. A number of private and public organizations have, through public statement and program investment, strongly endorsed the need for large-scale educational programs.
a Educating the public in genetics presents enormous challenges. Many people's views of how traits are inherited are inconsistent with Mendelian inheritance.20 New models of providing education and counseling to patients and other consumers are needed.E
thnic groups differ in their perceptions of disease origins and what should be done to avert disease.21-23 Moreover, identifying a genetic variant that has a much higher frequency in some ethnic groups than in others could have a stigmatizing effect on that group. In keeping with the overarching principles described in chapter 1, sensitivity to cultural differences is of paramount importance. Unfortunately, minorities are seriously under-represented in the field of genetics.Professional Education
Undergraduate and Graduate Medical Education.
The Task Force encourages the development of genetics curricula in medical school and residency training to enable all physicians to recognize inherited risk factors in patients and families, and appreciate issues in genetic testing and the use of genetic services. A committee of the American Society of Human Genetics has published a list of objectives for medical school courses and the skills and attitudes they should engender in medical students.24According to a 1995 survey by the Association of American Medical Colleges (AAMC), 68 of 125 four-year medical schools in the U.S. required genetics courses in their curricula (personal communication from Al Salas, Association of American Medical Colleges to Task Force, July 16, 1997). Although genetics is sometimes an integral part of other basic science courses in some other medical schools, the Task Force is concerned that genetics is not being taught adequately to all medical students. The AAMC survey also found that most genetics is taught in the first 2 (basic science) years of medical school. Consequently, many clinical aspects will not receive adequate attention. The Task Force is not suggesting that the courses be moved to the clinical years but that clinical departments pay greater attention to genetic issues.
As provider-patient communication is critical in offering genetic tests and counseling about them, consumers should be involved in the planning and implementation of new curricula in genetics. The Partnership for Genetic Services Pilot Program, just launched by the Alliance of Genetic Support Groups, and supported by public and private funds, has, as its goal, improving medical student and provider understanding, sensitivity, and competence in delivering genetic services. It will do this by exposing medical students and physicians-in-training to relevant community resource systems and illustrative presentations by consumers. Partnerships between consumers and clinical genetics providers, primary care practitioners, medical school faculty, and managed care administrators have been established.
Licensure and Certification. The likelihood that genetics will be covered in curricula will improve if relevant genetics questions are included in general licensure and specialty board certification examinations, and if correctly answering a proportion of the genetics questions is needed to attain a passing score. Medical school curriculum and residency review committees, which exist at both the local medical school and hospital levels and at the national level, define teaching content based on core material needed for clinical practice, recent advances, and questions on board examinations. Those who prepare board examinations, the National Board of Medical Examiners for medical students, and the various specialty boards for specialty certification, derive questions from material they think important, yet questions involving genetics are sparse and sometimes inappropriate. The American Council of Graduate and Medical Education (ACGME), is the umbrella organization for boards and residency review committees, and also contributes importantly to residency training content. The Task Force encourages ACGME, as well as residency review committees, to consider the importance of graduate training in genetics. The Task Force is pleased that the American Board of Obstetrics and Gynecology and the Society of Perinatal Obstetricians have acknowledged the importance of teaching genetics, including ethical aspects, by including questions on the basic obstetrics and gynecology exams, as well as on the subspecialty board exams of Maternal-Fetal Medicine.b
Traditionally, much medical school education and residency training occurred on the wards of hospitals. Those responsible for education and training have begun to recognize that most medical care is provided in ambulatory settings and that the delivery of care in those areas presents challenges for education. Genetic testing is a prime example. Moreover, teaching about genetic tests, including such issues as analytic and clinical validity, introduces students and residents to general problems of reliability and test sensitivity and specificity, which are important for a much wider range of clinical laboratory tests.
In a rapidly changing field such as genetics, curricula that focus on current discoveries and do not lay a basic framework will rapidly become obsolete. The Task Force is particularly concerned that underlying concepts of genetics are not adequately learned by all physicians.25 Equally important are the means of communicating genetic concepts and risks to patients. Although the tests will change, many aspects of patient-provider communication will not, although here, too, much research is beginning to explore the nature of these interactions.
Continuing Medical Education. The full beneficial effects of improving medical school and residency curricula in genetics will not be felt for many years. Consequently, improving the ability of providers currently in practice to offer and interpret genetic tests correctly is of paramount importance. The Task Force vigorously debated the question of whether this goal could best be accomplished by a "carrot" or "stick" approach. An early position taken by the Task Force was: "Some documentation of continuing education in the area of human and medical genetics should be required for physicians offering genetic tests, including primary care providers."26 (p.24) As the Task Force deliberated, it developed doubts as to whether continuing education could accomplish this goal and whether a requirement would accomplish the Task Force's objective. The Task Force was also concerned that such a requirement would be difficult to enforce. The need to demonstrate competence is discussed further in the next section, but the point the Task Force wishes to emphasize is the need for each specialty to recognize that all of those who are certified in that specialty appreciate the importance of genetics and genetic tests relevant to that specialty.
In addition to basic curricula already considered, the Task Force recommends that each specialty involved with the care of patients with disorders with genetic components should design its own curriculum for continuing education in genetics.Administrators and other nonphysician personnel who triage patients and/or make coverage or reimbursement decisions, such as those in managed care organizations, should also have knowledge of the benefits and risks of genetic testing.
Demonstrating Provider Competence
Hospitals and managed care organizations, on advice from the relevant medical specialty departments, should require evidence of competence before permitting providers to order predictive genetic tests defined as needing stringent scrutiny or to counsel about them.
Periodic, systematic medical record review, with feedback to providers, should also be used to ensure appropriate use of genetic tests.Prerequisites
. If hospitals and managed care organizations are to require evidence of competence, three prerequisites must be met. First, a mechanism must be in place for deciding which tests need evidence of competence. The Task Force believes that this should be one of the tasks of the proposed Secretary's Advisory Committee described in chapter 1. Second, competence must be defined. This can be accomplished by agreement between representatives of the non-genetic specialties involved in testing and of the genetics profession. Guidelines for establishing competence developed at the national level, e.g., by professional societies, which could be facilitated by the proposed Secretary's Advisory Committee, are ultimately preferable, but local agreements might be more readily reached when a test first becomes available. Third, easily accessible educational modules must be available to enable providers to gain competence. (We discuss some possibilities in the next section.) Unless continuing education opportunities are readily available, providers will be deterred from gaining sufficient knowledge of genetics to enable them to offer genetic tests appropriately to their patients.Although little precedent exists for asking for a demonstration of competence before ordering tests that will be performed primarily in ambulatory settings, there are several reasons why some predictive genetic tests (those requiring stringent scrutiny) should be ordered only by those with demonstrated competence. Some of these reasons are important primarily to the person being tested, some to the provider offering the test, and some to those paying for the test.
· People need to have sufficient information about the clinical validity of the test to decide whether the test is appropriate for them. Providers must be able to give them the requisite information.
· The implications of a positive or negative test result might influence people's decision to be tested. Providers must be aware of the implications and discuss them with the people considering testing.
· People's autonomy must be respected especially when procedures for avoiding the conception or birth of a child with a genetic disease are options following a positive test result. Atonomy is also crucial when the interventions in those with positive test results have not been proven to be safe and effective. Providers must recognize these situations, understand the need to respect autonomy, and be able to communicate information in the least directive manner possible.
· The results of some predictive genetic tests will indicate that relatives might be at risk of genetic disease. Providers must be prepared to discuss why and how the person tested should communicate with relatives and what the relatives should do.
· Providers could face legal liability if they order a test inappropriately or if they communicate results to relatives (except in extreme circumstances--see chapter 1) or unrelated third parties without the consent of the person tested.
· Third parties paying for the test, including managed care organizations, will not want to reimburse if the test has been ordered unnecessarily or inappropriately.
Enforcement. The Task Force does not favor requiring organizations to establish competence requirements. It believes self-interest, as just discussed, will lead many organizations to set them. Nor is it necessary for laboratories to request documentation of competence before they will perform a genetic test. Providers who work for organizations who do credential for genetic testing would place themselves in legal jeopardy if they ordered the test without having the credential. Providers who do not work with an organization that credentials, for instance a solo practitioner in private practice, might be competent to order genetic tests but will have no credential to present. In their survey, Burke and Press found that one quarter of respondents disagreed strongly with a suggestion that physicians should be required to undergo a brief certification in genetics before they could order susceptibility tests; less than 19% agreed strongly. The majority expressed moderate support for this position. (N. Press, personal communication, June 1997) As discussed in chapter 3, the laboratory does have a responsibility to determine from the requisition that the test is indicated, and that, when appropriate, informed consent has been obtained from the person to be tested or his or her legal guardian.
Medical record audits assure managed care and other organizations that providers are satisfying standards of care. The feedback given to providers also serves as a valuable reenforcement to what has previously been learned. Audits of records for frequently-ordered medical tests should be considered. The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and the National Committee for Quality Assurance (NCQA) should consider asking hospitals and other health care organizations to develop continuous quality improvement programs focusing on genetic testing.
Assisting Providers in Gaining Competence in Genetics
When organizations begin to require that providers have demonstrable competence in genetics, the means of acquiring that competence must be available. The American College of Medical Genetics is working with other specialties to set guidelines and standards to assist in the development of curricula. It responded to a request from the American Society of Clinical Oncologists to assist it setting up "train the trainer" modules for oncologists who can then train others in their specialty. ACMG would be responsive to requests from other organizations as well.
The Task Force endorses the recent establishment of a National Coalition for Health Professional Education in Genetics (NCHPEG) by the American Medical Association, the American Nurses Association, and the National Human Genome Research Institute
. The Coalition should work in consultation with non-genetic professional societies, such as the Association of American Medical Colleges, the American Council on Graduate and Medical Education, and genetic societies, such as the American College of Medical Genetics, the National Society of Genetic Counselors, the International Society of Nurses in Genetics, and appropriate consumer groups to encourage the development of core curricula in genetics. It should encourage input by consumers in the development of these curricula. In order to avoid duplication, the Coalition should serve as a registry and clearinghouse for, and disseminator of, information about various curricula and educational programs, grants, and training pilot programs in genetics education. It should encourage professional societies to track the effectiveness of their respective educational programs.The Task Force welcomes the interest of the Agency for Health Care Policy and Research (AHCPR) in helping the Coalition develop a research agenda in health education.
In 1994, the Maternal and Child Health Bureau (MCHB) of the Health Resources and Services Administration, through its Genetic Services Branch, began soliciting grant applications to strengthen genetics in primary care. Thus far nine programs have been funded and several more are expected to be funded in 1998.
c At least one educational module is available on the World Wide Web under MCH NetLink (http://www.ichp.ufl.edu?mch-netlink/). Others will appear shortly. Another MCHB grantee, the Council of Regional Networks for Genetic Services (CORN) has recently issued its Guidelines for Clinical Genetic Services for the Public's Health.26 MCHB has also asked CORN to prepare national guidelines that can be used for comprehensive followup care of children and families with rare metabolic disorders that can be used by purchasers and service providers in negotiating contracts with managed care plans. CDC has developed a Public Health Training Network that can be adapted to provide information about genetics. The network often employs satellite broadcasting to multiple receiving sites with phone communication from the sites to permit two-way communication. The Network also develops material for Internet presentations and self-study, computer-based training modules. A wide range of subjects have been presented, including basic epidemiology, specific disease management, immunizations, and managing laboratories under CLIA. The format includes lectures, panel discussions, and videos.A major problem in all educational endeavors is finding the "teachable moment," the time at which people, including health care providers, are receptive to new information and are most likely to retain it. These moments arise when providers are asked questions about genetic tests or when charts are flagged because the patient fulfills criteria for being offered a genetic test. Clearly, more people are asking providers questions about genetic tests. Computerized medical records or self-completing questionnaires (see box entitled Educational Module to Assist Physicians in Recognizing Genetic Risks) can generate flags to advise providers to offer genetic tests to people at risk. Printouts of background information, when flags are raised, could assist the provider.
A 1-800 hotline that providers (and the public, perhaps,) can call to learn more about specific genetic tests, including availability and indications for their use, should be established by NCHPEG or some of its governmental and private constituent organizations.
OTHER MODELS
Nursing
The overall time that physicians spend talking with patients on all subjects is usually less than the time that genetic counselors spend informing people about genetic tests and their implications. The nursing profession has recognized that nurses have much to offer in helping people appreciate the benefits and risks of genetic testing.27,28 Nurses can not only counsel (when trained) but also perform a wide range of activities in health care that genetic counselors are not qualified to do. Nurses are also in much greater supply. Nurses have been shown to be effective in providing education for testing for genetic susceptibility to cancer.29 Oncology nurses increasingly view themselves as genetic health care professionals.
d One nursing organization told the Task Force, "We see genetic education as core content in nursing education at both the undergraduate and advanced levels."e Nurses have played a large role in genetic counseling in the United Kingdom for many years.30 A study currently under way in the U.S. is comparing the ability of nurse practitioners and genetic counselors in educating and counseling about testing for genetic susceptibility to breast cancer. (G. Geller, work in progress) Nurses should be provided with additional education and training that can increase their effectiveness in providing education for people undergoing genetic testing.Community and Public Health
Although population-wide screening can be integrated into personal health care--prenatal screening in obstetrics provides a good example--different models have been used. In each case, screening has been undertaken because it permitted detection of many more at-risk subjects than would have been possible by using family histories. In this way, the opportunities for avoidance, prevention, or effective treatment are greater than waiting for symptoms to appear. For instance, diagnosing an older infant or child with phenylketonuria does little to prevent her retardation although it alerts the family to its risk of having additional children with that disease. Newborn screening, on the other hand, prevents retardation of the first child and all others who carry the disease-causing genotype (see appendix 5).
In many states, it is the responsibility of the hospital in which the baby is born to conduct screening. This model takes advantage of the fact that most babies are born in the hospital, making it easy to reach them. It is not advantageous once babies are discharged. Nevertheless, as testing for more inherited conditions become available and the safety and effectiveness of treating them neonatally is established, newborn screening could expand markedly.
Community-centered screening presents another model. Tay-Sachs carrier screening was originally organized at the community level; health care professionals who staffed the sites generally volunteered their time. The success of this effort depended on the cooperation of a cohesive community committed to screening. Nevertheless, not everyone in the ethnic group at risk came for screening and other methods had to be devised to reach them (see appendix 6). In the 1970s, and to a lesser extent today, sickle cell screening was performed at community sites and in health department clinics. For reasons discussed in appendix 6, this screening was not always a great success. Today, screening newborns for sickle cell anemia is part of many States' newborn screening programs.31 Sickle cell screening is succeeding in lowering morbidity and mortality from this disease among African-American children.32 Any effort to initiate community-based genetic screening must have the active support of the community. Particularly when minority communities are involved, the program must be sensitive to issues of discrimination and provide sufficient resources for education and counseling.
Many other disorders are spread throughout diverse communities and it would be a Herculean task to organize community-based screening. Screening could be offered in health department clinics, mobile vans, or other sites, but not all segments of the population are likely to utilize them. A greater chance of breaching confidentiality is possible at community and health department sites than in the privacy of the traditional provider-patient relationship. Informed consent might not always be obtained.33 Traditionally, health departments have been most involved in clinical care when there were well-accepted interventions (such as immunizations or tuberculosis control) without which the health of the public would be jeopardized. It might be difficult for public health personnel to appreciate that someone who refuses genetic screening is not jeopardizing the health of the public. Before these new models can be investigated, additional training of the personnel involved is necessary. Schools of nursing, public health, and social work need to strengthen their training programs in genetics.
fREFERENCES
1. Wilfond BS, Fost N: The cystic fibrosis gene: Medical and societal implications for heterozygote detection. JAMA 1990;263:2777-2783.
2. Mendlewicz J, Fleiss JL, Cataldo M, Rainer JD: Accuracy of the family history method in affective illness. Comparison with direct interviews in family studies. Archives of General Psychiatry 1975;32:309-314.
3. Kee F, Tiret L, Robo JY, et al: Reliability of reported family history of myocardial infarction. BMJ 1993;307:1528-1530.
4. Offit K, Brown K: Quantitating familial cancer risk: A resource for clinical oncologists. Journal of Clinical Oncology 1994;12:1724-1736.
5. Giardiello FM, Brensinger JD, Petersen GM, et al: The use and interpretation of commercial APC gene testing for familial adenomatous polyposis. New England Journal of Medicine 1997;336:823-827.
6. Hofman KJ, Tambor ES, Chase GA, Geller G, Faden RR, Holtzman NA: Physicians' knowledge of genetics and genetic tests. Academic Medicine 1993;68:625-631.
7. Geller G, Tambor ES, Chase GA, Hofman KJ, Faden RR, Holtzman NA: Incorporation of genetics in primary care practice. Will physicians do the counseling and will they be directive? Archives of Family Medicine 1993;2:1119-1125.
8. Geller G, Holtzman NA: A qualitative assessment of primary care physicians' perceptions about the ethical and social implications of offering genetic tesing. Qualitative Health Research 1995;5:97-116.
9. Holmes-Siedle MN, Rynanen M, Lindenbaum RH: Parental decisions regarding termination of pregnancy following prenatal detection of sex chromosome abnormality. Prenatal Diagnosis 1987;7:239-244.
10. Marteau TM, Plenicar M, Kidd J: Obstetricians presenting amniocentesis to pregnant women: Practice observed. Journal of Reproductive and Infant Psychology 1993;11:3-10.
11. Marteau TM, Drake H, Bobrow M: Counselling following diagnosis of a fetal abnormality: The differing approaches of obstetricians, clinical geneticists, and genetic nurses. Journal of Medical Genetics 1994;31:864-867.
12. Bernhardt BA: Empirical evidence that genetic counseling is directive: Where do we go from here? American Journal of Human Genetics 1997;60:17-20.
13. Michie S, Bron F, Bobrow M, Marteau TM: Nondirectiveness in genetic counseling: An empirical study. American Journal of Human Genetics 1997;60:40-47.
14. Kessler S: Psychological aspects of genetic counseling. VII. Thoughts on directiveness. Journal of Genetic Counseling 1992;1:9-17.
15. Clarke A: Is non-directive genetic counselling possible? The Lancet 1991;338:998-1001.
16. Kevles DJ: In the name of eugenics. New York, Alfred A. Knopf Inc. 1985.
17. Reilly P: The surgical solution: A history of involuntary sterilization in the United States. Baltimore, The Johns Hopkins University Press; 1991.
18. Bernhardt BA, Pyertiz RE: The economics of clinical genetics services. III. Cognitive genetics services are not self-supporting. American Journal of Human Genetics 1989;44:288-293.
19. Surh LC, Wright PG, Cappelli M, et al: Delivery of molecular genetic services within a health care system: Time analysis of the clinical workload. American Journal of Human Genetics 1995;56:760-768.
20. Richards M: Lay and professional knowledge of genetics and inheritance. Public Understanding of Science 1996;5:217-230.
21. Angel R, Thoits P: The impact of culture on the cognitive structure of illness. Cultural Medical Psychiatry 1987;11:465-494.
22. Punales-Morejon D, Penchaszadeh VB: Psychosocial aspects of genetic counseling: Cross-cultural issues. Birth Defects 1992;28:11-15.
23. Dibble SL, Vanoni JM, Miaskowski C: Women's attitudes toward breast cancer screening procedures: Differences by ethnicity. Women's Health Issues 1997;7:47-54.
24. American Society of Human Genetics Information and Education Committee: Report from the ASHG Information and Education Committee: Medical school core curriculum in genetics. American Journal of Human Genetics 1995;56:535-537.
25. Task Force on Genetic Testing: Interim principles. Available at www.med.jhu.edu/tfgtelsi 1996.
26. Council of Regional Networks for Genetic Services (CORN): Guidelines for Clinical Genetic Services for the Public's Health. 1997;First Edition, CORN, Atlanta GA.
27. Monsen RB: Nursing takes leading role in genetics education: Coalition formed to increase provider awareness on genetics technologies. American Nurse 1996;28:11
28. Anderson GW: The evolution and status of genetics education in nursing in the United States 1983-1995. Image The Journal of Nursing Scholarship 1996;28:101-106.
29. Lerman C, Biesecker B, Benkendorf JL, et al: Controlled trial of pretest education approaches to enhance infomed decission-making for BRCA1. Journal of the National Cancer Institute 1997;89:148-157.
30. Williams A: Genetic counseling. A nurse's perspective. In Clarke A (ed): Genetic Counseling. Practice and Principles. New York, Routledge; 1994:44-62.
31. Hiller EH, Landenburger G, Natowicz MR: Public participation in medical policy making and the status of consumer autonomy: The example of newborn screening programs in the United States. American Journal of Public Health 1997;87:1280-1288.
32. Vichinsky E, Hurst D, Earles A, Kleman K, Lubin B: Newborn screening for sickle cell disease: Effect on mortality. Pediatrics 1988;81:749-755.
33. Farfel MR, Holtzman NA: Education, consent, and counseling in sickle cell screening programs: Report of a survey. American Journal of Public Health 1984;74:373-375.
CHAPTER 5. GENETIC TESTING FOR RARE INHERITED DISORDERS
The vast majority of single-gene (Mendelian) disorders are rare, occurring less often than 1 in 10,000 live births. Exceptions are sickle cell anemia, cystic fibrosis, thalassemia, and Tay-Sachs disease in some populations, and heterozygous familial hypercholesterolemia, Duchenne muscular dystrophy, and the hemophilias more generally. Phenylketonuria, for which newborns are routinely screened, occurs in slightly less than 1 in 10,000 births. Most of the several thousand other known inherited diseases occur much less frequently, but their combined incidence is by no means rare. Between 10 and 20 million Americans may suffer from one of the several thousand known rare diseases over their lifetimes.1(p. xiii) With the discovery of the role of inherited mutations in common diseases, such as breast and colon cancer and Alzheimer disease (albeit in a small proportion of affected people), the Task Force is concerned that research might shift away from the multitude of rare diseases. Commercial genetic test developers, for instance, expend a greater effort on the common, complex disorders than on rare ones (see table 3, appendix 3). The development and maintenance of tests for rare genetic diseases must continue to be encouraged.
Congress recognized the need to provide incentives for the development of drugs for rare diseases when it passed the Orphan Disease Act in 1983.2 To stimulate research and development, it granted a 7-year period of market exclusivity for unpatented drugs, a tax credit to offset the cost of drug development (the tax credit expired in 1994), and government grants and contracts to help defray costs of clinical studies. Over 300 grants have been awarded, primarily to support the development of drugs and biologics. (Personal communication, Dr. John V. Kelsey, Office of Orphan Products Development, FDA, February 22, 1996) In 1988, Congress added medical devices, authorizing government grants and contracts for "defraying the costs of developing medical devices for rare diseases or conditions."3 Devices now account for about 10% of all orphan products receiving assistance under the Act.
As part of the Safe Medical Devices Act of 1990, Congress enacted the Humanitarian Device Exemption "to encourage the discovery and use of devices intended to benefit patients in the treatment and diagnosis of diseases or conditions that affect fewer than 4,000 individuals in the United States."4 The incentive to device manufacturers is temporary authorization to market the device without meeting the effectiveness requirements of FDA. The exemption lasts for 18 months, although it can be renewed for up to 5 years. During the period of the exemption, the manufacturer cannot obtain a profit on device sales; the device must receive pre-market approval before a profit mark-up can be included in the price. In addition to the special incentives under these Acts, approximately 20% of the National Institutes of Health (NIH) budget funds research that is related to rare diseases, of which about 90-95% are inherited. (Personal communication, Steven Groft, Director Office of Rare Diseases, NIH, October-November 1996)
There is no uniform definition of a rare disease. The Orphan Drug Act (ODA) defines orphan disease as one affecting less than 200,000 persons in the U.S., or approximately 1 in 1,250 Americans. For devices (which include genetic tests), the 1988 ODA Amendments define rare disease as "any disease or condition that occurs so infrequently in the United States that there is no reasonable expectation that a medical device...will be developed without [financial] assistance."
a As already noted, the Humanitarian Device Exemption of the Safe Medical Devices Act of 1990 applies to diseases or conditions that affect fewer than 4,000 persons in the United States (1 in 62,500 Americans). It is silent on what constitutes a disease or condition (e.g., whether rare variants of a common genetic disease constitute a separate disease, or whether carriers are excluded). The carrier (heterozygote) frequency for autosomal recessive disorders with an incidence of 1 in 10,000 is 1 in 50.Of great concern to the Task Force is the dissemination of information related to the diagnosis and management of rare diseases, the continuing availability of tests for their diagnosis and for predicting risk of future disease, and, finally, the quality of laboratories performing genetic tests for rare diseases. We consider these topics in turn in the remainder of this chapter.
DISSEMINATION OF INFORMATION ABOUT RARE DISEASES
Research Activity
The NIH Office of Rare Diseases (ORD), founded in 1994, maintains a database of clinical studies involving rare diseases that are funded by NIH. At the end of 1996, approximately 300 studies were contained in the database. ORD plans to expand the database to include clinical research supported by private organizations, including the biotechnology and pharmaceutical industries. When fully operational, the database will contain abstracts of studies, enrollment criteria, and the names of principal investigators and how to contact them. The database is available to patients, providers, and other researchers on the World Wide Web at http://rarediseases.info.nih.gov/pages. In the future, people may be able to contact principal investigators of clinical studies through the databases. ORD would also like to coordinate rare disease research by the establishment of an information center, which would also respond to inquiries about rare genetic disorders. Funds have been authorized but not appropriated.
The Metabolic Information Network (MIN) (Dallas, Texas) is a registry containing medical information on approximately 10,000 living and deceased patients with any one of 86 metabolic disorders. Funded originally by the National Institute of Child Health and Human Development, MIN currently receives most of its support from pharmaceutical companies. Through MIN, an investigator doing research on a particular disease can locate other investigators doing related research. Names of patients are not included in the registry and requests for investigator-to-investigator contact are reviewed by a scientific advisory board.
A major concern of the Task Force is that as tests to diagnose and, in many cases, to predict, rare diseases are developed, data will not be systematically compiled on their clinical, as well as analytical validity. A comprehensive system to collect data on rare diseases must be established. As discussed in chapter 2, the Center for Disease Control and Prevention (CDC) can and should play a role in coordinating data collection from multiple sources to facilitate the review of new genetic tests, particularly for rare diseases. Multiple sources will almost always be needed to validate tests for rare diseases.
CDC and ORD should work closely to develop the appropriate data-gathering and monitoring systems to assess the validity of genetic tests for rare diseases.Finding Information on the Interpretation of Clinical Findings
Some rare genetic diseases present with unusual symptoms or signs, making diagnosis relatively easy for knowledgeable physicians. Many rare inherited metabolic disorders present with commonly encountered problems for which the usual explanation is not a rare disease.5 When the clinical problem persists or recurs despite treatment, health care providers must be aware that a rare disease could be the explanation. Prompt recognition can often save the patient's life by leading to initiation of effective therapy before irreversible damage occurs. Many of these metabolic disorders appear in infants and children; early diagnosis can alert the parents to their risk of having another affected child. Several tests can be used predictively for prenatal diagnosis. Carrier testing in collateral relatives is often possible.
Unfortunately, the diagnosis of rare diseases is often delayed. One reason for the delay is inaccessibility of information.
Physicians who encounter patients with symptoms and signs of rare genetic diseases should have access to accurate information that will enable them to include such diseases in their differential diagnosis, to know where to turn for assistance in clinical and laboratory diagnosis, and to locate laboratories that test for rare diseases. The commonly encountered symptoms and signs with which rare diseases present and the process of evaluating them should be taught to medical students and residents. It would be too much to expect health care providers to retain information on all the unusual presentations, but they should be taught where to seek information. Although textbooks and medical journals are the classical starting points, and referrals to specialists may help, computerized databases in which a user could search by the patient's presenting finding would be more expeditious and effective. Most available information is organized by disease, not by presenting findings. The National Organization of Rare Diseases, Inc. (NORD) publishes The Physician's Guide to Rare Diseases, which includes an atlas of visual diagnostic signs. NORD also maintains the rare disease database containing entries on over 1,100 rare diseases. The database is logically organized by a description of the disorder, symptoms, causes, affected population, related disorders, diagnostic procedures, status of treatment (investigational or standard of care), resource referral for further information and support, and references from peer-reviewed medical literature. The database is available on the World Wide Web at http://www.nord-rdb.com/~orphan.Although primarily providing information to researchers, the Metabolic Information Network can provide information to physicians on over 200 metabolic disorders.
Once diagnoses are made, patients and/or their families often want written information about the diseases. In a survey of 270 physicians conducted about 10 years ago, 42% were unable to find printed information to distribute to their patients with rare diseases.1 NORD's database on rare diseases has since been made available to consumers. NORD also maintains a Patient Services Department, one of whose functions is to help affected individuals and families in need of accessing services. The Department also maintains a confidential patient registry.
Information about individual disorders, particularly for consumers, is also available through individual genetic support organizations, which can be located through NORD (Washington, DC) or the Alliance of Genetic Support Groups (Chevy Chase, Maryland).
Finding Clinical Diagnostic Laboratories
Because of the rarity of many diseases, only one or a few laboratories in the United States, or the world, accurately perform tests for them. This raises the problem of how physicians caring for patients will be able to identify these laboratories in time to benefit patients who present with acute illness.
The Helix Directory of Medical Genetics Laboratories, supported by the National Library of Medicine, lists approximately 300 laboratories that perform tests on over 480 genetic diseases. Helix began by listing laboratories performing DNA-based tests including fluorescent in situ hybridization (FISH), but will extend to biochemical tests in the future. As of July 1997, Helix has 4,500 registered users and receives 150 requests per day. (Personal communication, Maxine L. Covington, Helix Directory Manager, July 23, 1997) Helix provides information by phone and fax, but it is encouraging inquiries via the World Wide Web at http://www.hslib.washington.edu/helix. As many of the laboratories entered in the database do not want to be contacted directly by patients, passwords for entry to the database are available only to health care providers. Consequently, Helix is not listed in NORD's databases.
Through ORD's database on clinical research studies, physicians can get help in the diagnosis of patients in whom they suspect particular rare diseases. To maintain and expand its database, ORD should identify laboratories worldwide that perform tests for rare genetic diseases, the methodology employed, and whether the tests they provide are in the investigational stage, or are being used for clinical diagnosis and decision making.
Need for Coordination
The Task Force is concerned that there might be some unnecessary duplication of effort in compiling databases while, at the same time, some diseases or laboratories offering tests will not be included. In addition to the databases mentioned so far, several other organizations, including the Alliance of Genetic Support Groups, some of its member organizations, and other independent genetic disease interest groups maintain databases and, in some cases, patient registries. The American Academy of Pediatrics provides information periodically on newborn screening and other disorders. The Society for Inherited Metabolic Disorders is compiling information for providers about diagnostic evaluations of rare disorders, and ACMG is developing databases on tests that should be used to diagnose specific disorders.
In order to avoid redundancy and to use the expertise of these organizations more efficiently, NIH should assign its Office of Rare Diseases (ORD) the task of coordinating these efforts and provide ORD with sufficient funds to fulfill the Task Force's recommendations on rare diseases. ORD should periodically report to the proposed Secretary's Advisory Committee on the status of these activities. With CDC playing a greater role in genetics, it should be closely involved in activities in this area.
ENSURING CONTINUITY AND QUALITY OF TESTS FOR RARE DISEASES
The clinical diagnostic tests for some rare diseases are available only from laboratories that are primarily engaged in research. Some of these laboratories perform clinical tests at no cost to the patient and with the primary purpose of furthering their own research. This raises two questions: First, what happens to the availability of the test for clinical diagnostic purposes when the laboratory (or laboratories) performing the assay ceases to do so because it switches to other research projects or for other reasons? Second, as discussed in the concluding section, how can the quality of clinical test performance be assured in laboratories engaged primarily in research?
Research laboratories that were offering genetic tests for rare diseases will cease performing them as they complete their investigations and move on to other areas of interest. This is particularly a problem for the continued availability of clinical tests when only one research laboratory performed the test. It is not unlikely, however, that as progress on a given rare diseases is made, all of the research laboratories offering tests will move on to solve other problems.
The Task Force considered the transitioning problem at great length. It rejected the possibility of creating central or regional laboratories that could perform a wide range of tests for rare diseases because assembling the necessary expertise for performing and interpreting all of the tests under one roof would be difficult or impossible. For the same reason, it rejected transfer of these tests to large mega-test commercial laboratories that might be willing to add on tests for rare diseases if they could cover costs. The Task Force also considered whether agencies funding research that included the development and offering of tests for rare diseases should be asked to allocate a small part of the grant or contract they awarded to enable the investigator to transfer the test to a service laboratory just before funding for the research terminated. This might discourage investigators from applying for grants if they were reluctant to take on this responsibility. Agencies funding research might also be reluctant to use funds to establish service activities. They might also have concerns about the quality of the tests being offered as a service.
The Task Force is not convinced that the transitioning problem is insurmountable. One possibility is that a laboratory that was offering genetic tests as part of its research, but on which clinical decisions were being made, procure CLIA certification (see below) and serve as a service laboratory, recovering its costs for the test by instituting charges for it. Another possibility is for the research laboratory to transfer the testing capabilities to the clinical diagnostic laboratory in its institution. The proximity of the expert investigator could facilitate a smooth transition and ensure the test would be performed and interpreted properly. A third possibility is that the test be transferred to a research laboratory elsewhere that is willing to perform the test as a service. In this case, mechanisms are needed to ensure that providers know where to obtain the test. Whichever alternative is adopted, the test should undergo some form of external review before transition to a service.
The NIH Office of Rare Diseases should have the lead responsibility in ensuring the continued availability of safe and effective tests for rare diseases when it learns that a test will cease being offered. Funds to enable it to accomplish this task should be available.
Laboratories should notify ORD about impending cessation of their testing so that provisions for a transition to other laboratories can be made. ORD should, in turn, notify other laboratories when a demonstrably safe and effective genetic test ceases to be available and make every effort to get another laboratory to perform it. If this fails, ORD should notify the other organizations with whom it coordinates, as well as the proposed Secretary's Advisory Committee.
ENSURING THE QUALITY OF GENETIC TESTS FOR RARE DISEASES
Neither the clinical nor the laboratory diagnosis of rare inherited diseases is easy. If clinicians do not mention the possibility of a rare disorder when they order clinical laboratory tests, the laboratory might not test for them. Clinical laboratories, too, might misinterpret abnormal findings, often neglecting rare disorders in favor of more common situations, such as poisoning. Some clinical laboratories do not have the equipment or expertise to diagnose a rare disorder, but clinicians might not realize it. (That is one reason why directories of qualified laboratories, as will be discussed further, are so important.) Many rare disorders will be diagnosed only by special laboratories accustomed to looking for rare diseases and having the equipment and expertise to do so.
Some genetic tests for rare diseases have been developed in research laboratories under grants. In accordance with current law, the Task Force recommends that any laboratory performing any genetic test on which clinical diagnostic and/or management decisions are made should be certified under CLIA. Research laboratories that are not currently providing genetic test results to providers or patients but that plan to do so in the future must register under CLIA. Once a laboratory registers, it does not have to wait for a survey (see chapter 3) before performing clinical tests.
Some research laboratories have complained of the difficulty and expense of obtaining CLIA approval for tests that constitute a small part of their activity and will only be performed occasionally. A laboratory performing 2,000 or fewer tests a year can register for $100 and obtain certification for $300 (including onsite inspection for its first 2 years.)
bThe Task Force recognizes the important contribution that research laboratories make to clinical testing, particularly for rare diseases. The type of skills that are needed for research, including a willingness to modify experimental conditions, are not necessarily the skills for maintaining the quality of a service laboratory, in which consistency of performance ensures reliability.
Research laboratories that provide physicians with results of genetic tests, which may be used for clinical decision making, must validate their tests and be subject to the same internal and external review as other clinical laboratories. Nevertheless, the proposed genetics subcommittee of CLIAC should consider developing regulatory language under the proposed genetics specialty that is less stringent, but does not sacrifice quality for laboratories that only occasionally and in small volume perform tests whose results are made available to health care providers or patients.cOf great concern to the Task Force, discussed at length in chapter 3, is whether certification under CLIA will ensure the quality of genetic tests, particularly those for rare genetic diseases. The creation of a subspecialty of genetics under CLIA will greatly improve the situation. Many tests for rare disorders are biochemical. The quality of performance of these tests would be ensured if they were included under a genetics specialty.
Directories of laboratories that perform tests for rare genetic diseases should indicate whether or not the laboratory is CLIA-certified and whether it has satisfied other quality assessment and proficiency assessments, such as those provided by CAP and ACMG. Directors of these laboratories are encouraged to participate in these programs or other programs of at least comparable quality that may be established.
The Task Force is concerned that third-party payers, including managed care organizations will not recognize that tests for rare diseases can only be performed in certain highly-specialized laboratories. Patients will be misdiagnosed and harmed unless these laboratories are used. The Society of Inherited Metabolic Diseases is preparing a list of laboratories qualified to perform tests for several rare diseases. The Helix database should also indicate whether the laboratories listed in it are CLIA-registered and/or certified. When the proposed genetics specialty is established, the directories should indicate whether the laboratory performing genetic tests is certified in that specialty or the appropriate subspecialty.
REFERENCES
1. National Commission on Orphan Diseases: Report of the National Commission on Orphan Diseases. 1989;(Abstract)
2. Public Law 97-414: 1995;U.S.C. Sec 360aa et:(Abstract)
3. Public Law 100-290: Orphan Drug Amendments of 1988. 1995;U.S.C. Sec 360cc(a):(Abstract)
4. Public Law: Safe Medical Devices Act of 1990. 1995;U.S.C. Sec 360j(m):(Abstract)
5. Holtzman NA: Rare diseases, common problems: Recognition and management. Pediatrics 1978;62:1056-1060.
6. Shoemaker JD, Lynch RE, Hoffmann JW, Sly WS: Misidentification of propionic acid as ethylene glycol in a patient with methylmalonic acidemia. Journal of Pediatrics 1992;120:417-421.
7. Woolf AD, Wynshaw-Boris A, Rinaldo P, Levy HL: Intentional infantile ethylene glycol poisoning presenting as an inherited metabolic disorder. Journal of Pediatrics 1992;120:421-424.
CHAPTER 6. SUMMARY AND CONCLUSIONS
The Task Force recommends that the Secretary of Health and Human Services appoint an advisory committee on genetic testing to be instrumental in implementing the recommendations of this Task Force. The advisory committee or its designate should establish a system for determining which genetic tests require stringent scrutiny. If a test is likely to be used to predict future disease in healthy people, it is a candidate for stringent scrutiny, but not all predictive tests will necessarily require such scrutiny and other criteria are needed as well.
The Task Force wishes to highlight the following recommendations and to indicate the organizations primarily responsible for facilitating them:
(1) Protocols for the development of genetic tests that can be used predictively must receive the approval of an institutional review board (IRB) when subject identifiers are retained and when the intention is to make the test readily available for clinical use. OPRR in cooperation with the proposed Secretary's Advisory Committee is primarily responsible.
(2) Test developers must submit their validation and clinical utility data to external review as well as to interested professional organizations in order to permit informed decisions about routine use. Independent review should take place at both the local level (e.g., academic center or company), and at the national level by professional societies, consensus panels, Federal agencies and other organizations, before new tests become available for noninvestigational clinical use. The proposed Secretary's Advisory Committee should coordinate national efforts.
(3) The Task Force urges the newly created genetics subcommittee of the Clinical Laboratory Improvement Advisory Committee to consider the creation of a specialty of genetics that would encompass all predictive tests that satisfy criteria for stringent scrutiny. If only a subspecialty for DNA/RNA-based tests is feasible, the subcommittee must then address how to ensure the quality of laboratories performing nonDNA/RNA predictive genetic tests. The agencies primarily responsible for administering CLIA, HCFA and CDC, should take the lead in implementing this recommendation.
(4) The Task Force encourages the development of genetics curricula in medical school and residency training. In addition to these basic curricula, each specialty involved with the care of patients with disorders with significant genetic components should design relevant curricula for continuing education in genetics. Schools of nursing, public health, and social work need to strengthen and expand their training programs in genetics. The newly created National Coalition for Health Professional Education in Genetics should greatly facilitate improving professional education in genetics.
(5) Hospitals and managed care organizations should require evidence of competence before permitting providers to order predictive stringent scrutiny genetic tests or to counsel about them. Implementation is at the local level. If accrediting organizations include a review of the management of selected genetic tests as part of their accreditation, there will be greater stimulus for local organizations to ensure quality.
(6) Physicians who encounter patients with symptoms and signs of rare genetic diseases should have access to accurate information that will enable them to include such diseases in their differential diagnosis, to know where to turn for assistance in clinical and laboratory diagnosis, and to locate laboratories that test for rare diseases. The quality of laboratories providing tests for rare diseases must be assured, and a comprehensive system to collect data on rare diseases must be established. The NIH Office of Rare Diseases should play a coordinating role. The genetics subcommittee of CLIAC should examine means of assuring the quality of laboratories performing tests for rare diseases.
These and the many other principles and recommendations of the Task Force presented herein will help ensure that genetic testing will be provided safely and effectively and that tests for rare diseases will be more widely available but used appropriately. The Task Force concludes that with implementation of these recommendations, genetic testing will continue to flourish.
The Climate Change and Public Health Law Site
The Best on the WWW Since 1995!
Copyright as to non-public domain materials
See DR-KATE.COM for home hurricane and disaster preparation
See WWW.EPR-ART.COM for photography of southern Louisiana and Hurricane Katrina
Professor Edward P. Richards, III, JD, MPH - Webmaster
Provide Website Feedback - https://www.lsu.edu/feedback
Privacy Statement - https://www.lsu.edu/privacy
Accessibility Statement - https://www.lsu.edu/accessibility